Method for preparing hydroxylamine through nitro-reduction
A technology of phenylhydroxylamine and hydroxylamine, which is applied in the field of preparation of hydroxylamine by reduction of nitro groups, can solve the problems of harsh preparation reaction conditions and inapplicability to large-scale production, and achieve the effects of mild reaction conditions, cheap raw materials, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
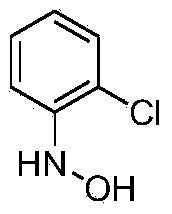
[0013] The synthesis of embodiment 1 N-(2-chlorophenyl) hydroxylamine
[0014]
[0015] Add 48.2g of o-chloronitrobenzene (0.3mol, 98%, 1.0eq), 360g of ethanol, 360g of 1,2-dichloroethane, and 8g of Raney Ni into a 1L jacketed kettle equipped with mechanical stirring, a thermometer and an air duct , 2g of tetrabutylammonium bromide, start stirring; raise the temperature to 15°C, start to add hydrazine hydrate 46.9g (0.75mol, 80%, 2.5eq) dropwise, after the dropwise addition, continue to keep warm for 4h; after the reaction is complete, filter the reaction solution , the filtrate was rotary evaporated, and the residue of the kettle was recrystallized by adding ethanol to obtain 32.3 g of N-(2-chlorophenyl) hydroxylamine, with a yield of 75%.
Embodiment 2
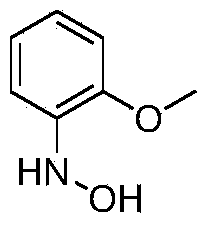
[0016] The synthesis of embodiment 2 N-(4-methoxyphenyl) hydroxylamine
[0017]
[0018] Add 46.8g (0.3mol, 98%, 1.0eq) of 4-methoxynitrobenzene, 720g of ethanol, 10g of Raney Ni, and tetrabutylammonium bromide into a 1L jacketed kettle equipped with mechanical stirring, a thermometer and an air duct 1g, start stirring; raise the temperature to 25°C, start to dropwise add 37.5g of hydrazine hydrate (0.6mol, 80%, 2.0eq), after the dropwise addition, continue to keep warm for 4h; Ethanol was added for recrystallization to obtain 27.1 g of N-(4-methoxyphenyl) hydroxylamine with a yield of 65%.
Embodiment 3
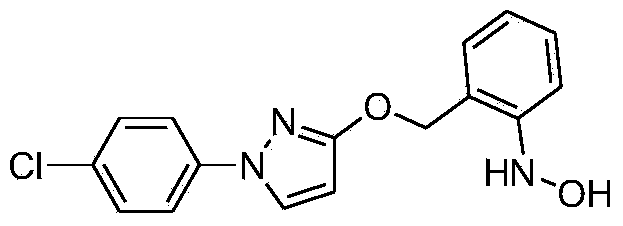
[0019] Example 3 Synthesis of N-hydroxyl-N-2-[(N-p-chlorophenyl)-3-pyrazolyloxymethyl]aniline
[0020]
[0021] Add 100.9g (0.3mol, 98%, 1.0eq) of 2-[N-p-chlorophenyl]-3-pyrazolyloxy]nitrobenzene into a 1L jacketed kettle equipped with mechanical stirring, a thermometer and an air duct , 720g of isopropanol, 15g of Raney Ni, 0.8g of tetrabutylammonium bromide, start stirring; raise the temperature to 15°C, start to add 75g of hydrazine hydrate (0.75mol, 50%, 2.5eq) dropwise, after the dropwise addition is completed, continue to keep warm for 4h After the reaction is complete, the reaction solution is filtered, the filtrate is rotary evaporated, and the residue of the kettle is recrystallized by adding ethanol to obtain a light yellow solid product N-hydroxyl-N-2-[(N-p-chlorophenyl)-3-pyrazolyloxymethyl Base] aniline 80.5g, yield 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com