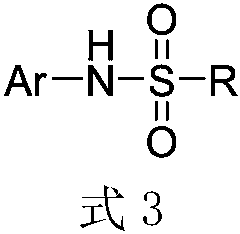
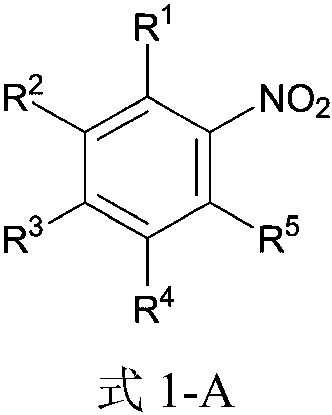
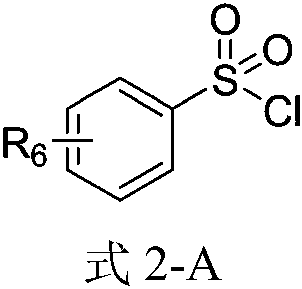
Method for ultrasound-assisted synthesis of N-arylsulfonamide
An aryl sulfonamide, ultrasonic-assisted technology, applied in the preparation of sulfonic acid amide, organic chemistry, etc., can solve the problems of complex reaction conditions, inability to prepare N-aryl fatty sulfonamide, and limit industrial application, and achieve reaction efficiency High, low cost, low price effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Preparation of N-phenylbenzenesulfonamide (N-phenylbenzenesulfonamide):
[0052]In a 25mL round bottom flask, add 1.23g of nitrobenzene, 2.64g of benzenesulfonyl chloride, 1.96g of iron powder, and 10mL of water in sequence, and react the resulting mixture in a 35W / 40KHz ultrasonic stirring (600 rpm) reaction device for 60 minutes . The reactant was extracted with ethyl acetate and recrystallized to obtain 2.26 g of the corresponding N-phenylbenzenesulfonamide, with a yield of 97%.
Embodiment 2
[0054] Replace 35W / 40KHz ultrasonic stirring with 45W / 40KHz ultrasonic stirring:
[0055] In a 25mL round bottom flask, add 1.23g of nitrobenzene, 2.64g of benzenesulfonyl chloride, 1.96g of iron powder, and 10mL of water in sequence, and react the resulting mixture in a 45W / 40KHz ultrasonic stirring (600 rpm) reaction device for 60 minutes . The reactant was extracted with ethyl acetate and recrystallized to obtain 2.26 g of the corresponding N-phenylbenzenesulfonamide, with a yield of 97%.
Embodiment 3
[0057] Replace 35W / 40KHz ultrasonic stirring with 25W / 40KHz ultrasonic stirring:
[0058] In a 25mL round bottom flask, add 1.23g of nitrobenzene, 2.64g of benzenesulfonyl chloride, 1.96g of iron powder, and 10mL of water in sequence, and react the resulting mixture in a 25W / 40KHz ultrasonic stirring (600 rpm) reaction device for 60 minutes . The reactant was extracted with ethyl acetate and recrystallized to obtain 1.98 g of the corresponding N-phenylbenzenesulfonamide, with a yield of 85%.
[0059] From Examples 1, 2 and 3, it can be known that adjusting the power of ultrasound can affect the yield of the product. Research has found that the yield of the product can be further improved at a power of 35W or above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com