Main chain type azobenzene polymer and preparation method thereof
A technology of azobenzene and polymers, applied in the field of polymer material synthesis, can solve the problems of low stability of raw materials, harsh reaction conditions, and low conversion rate, and achieve less side reactions, mild reaction conditions, and low waste rate low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
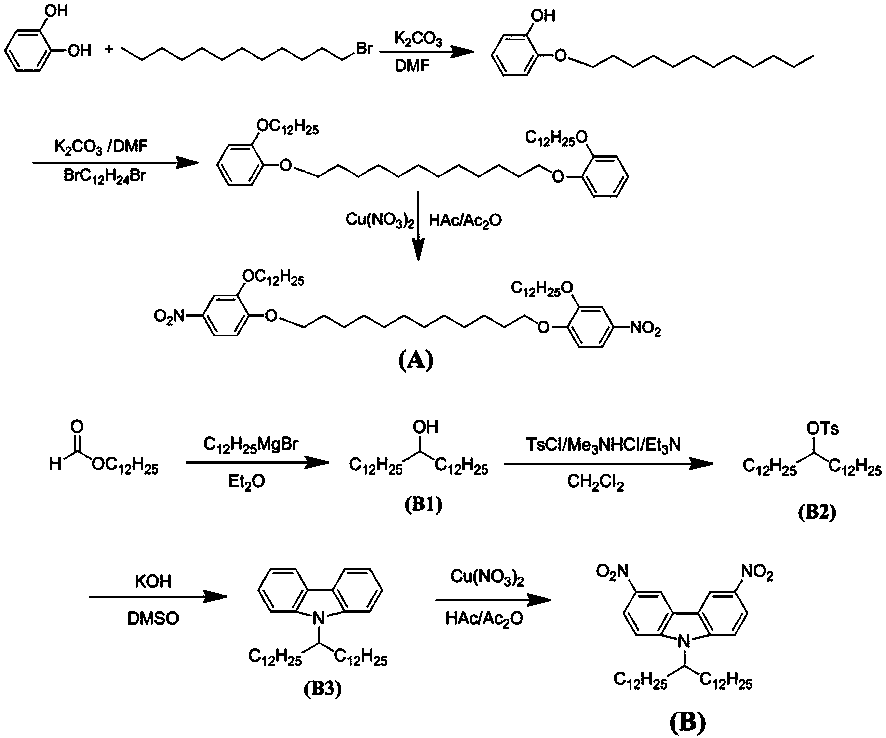
[0048] Example 1. Synthesis of main chain azo polymers (PA, PB, PC)
[0049] figure 1 It is a schematic flow sheet of the present invention for preparing nitro group-containing monomers, according to the appendix figure 1 :
[0050] Synthesis of Nitro-Containing Monomer Compound A
[0051] 14.0 g of potassium carbonate and 200 mL of N, N -Dimethylformamide was stirred at 80°C for 2 hours, and 12.0 g of catechol was added to the above solution, followed by stirring for 1 hour. Then 20.0 g of 1-bromododecane was added dropwise to the above mixed solution, and a small amount of potassium iodide was added, and stirred at 80 °C for 8 hours. After that, the reaction solution was added to a 500 mL ice-salt bath beaker, Stir, let stand, and filter with suction to obtain crude product. The crude product was separated by column chromatography with an eluent of petroleum ether / ethyl acetate = 20 / 1 to obtain a pure white product. Take 8 g of the above white product, 3.2 g of 1,12-d...
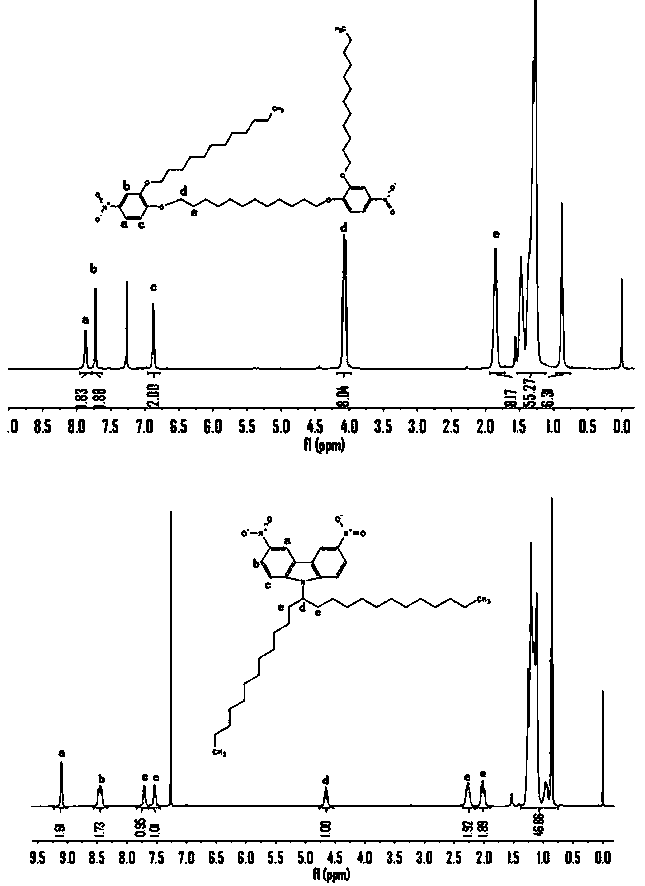
Embodiment 2
[0071] Example 2. Photoinduced cis-trans isomerization of main chain azo polymers (PA, PB, PC)
[0072] The above-mentioned non-conjugated polymer PA and conjugated polymers PB and PC were dissolved in tetrahydrofuran to prepare a polymer tetrahydrofuran solution with a concentration of 0.033 mg / mL, and the solution was irradiated with ultraviolet light to study the photoinduced cis-trans isomerization of each polymer.
[0073] attached Figure 7 , attached Figure 8 , attached Figure 9 are the photo-induced cis-trans isomerization spectra of the non-conjugated polymer PA and the conjugated polymers PB and PC, respectively.
[0074] like Figure 7 As shown, the tetrahydrofuran solution (concentration of 0.033 mg / mL) of the non-conjugated polymer PA undergoes cis-trans isomerization under the irradiation of 365 nm ultraviolet light. After 35 seconds of irradiation, the azo-trans isomerization of the polymer The structure is basically in balance. Then 435 nm ultraviolet ligh...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com