Method for preparing ferric oxide red pigment by using nitryl chloride tail gas
A technology of nitroxyl chloride tail gas and iron oxide red, which is applied in the direction of iron oxide, iron oxide/iron hydroxide, etc., to achieve the effect of reducing environmental pollution, saving energy and reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
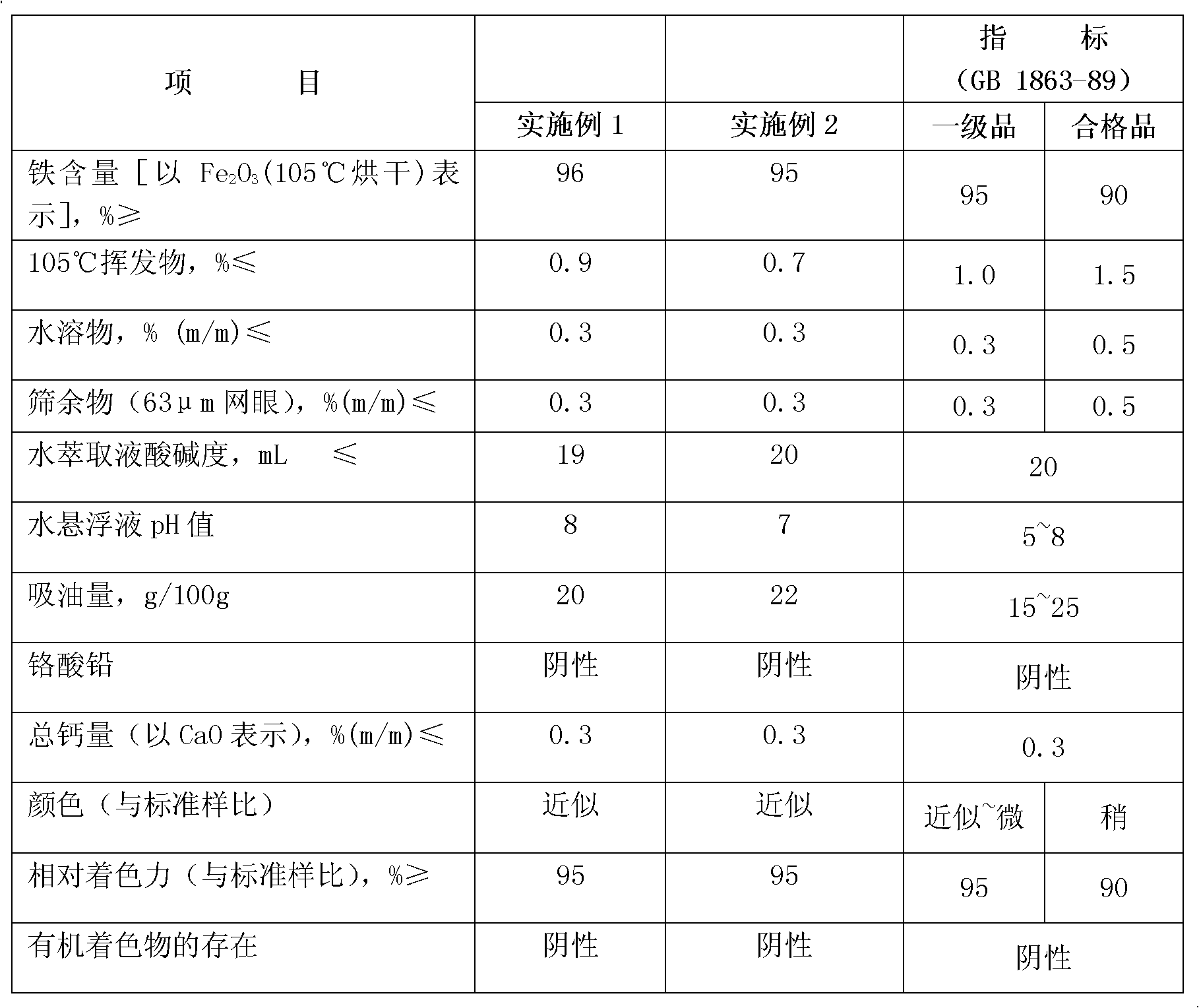
[0021] Add 240g of chemical iron sludge (from Zhejiang Changshan Changsheng Chemical Industry Co., Ltd.) into the water absorption tank of nitroxyl chloride, heat it with steam at 90-100°C, stir for 30 minutes to dissolve the iron sludge, and measure Fe in the solution. 3+ After the content reaches 110g / L, filter. The filtrate is transferred to the sedimentation tank, and an appropriate amount of ammonia water is added to control the pH value to 9, so that all the ferric iron is formed into ferric hydroxide to precipitate, and the solid and liquid are separated by a centrifugal separator, and the solid ferric hydroxide is put into a heating kiln for heating at 110°C In 10 minutes, the solid turned red and decomposed to obtain 311g of pure iron oxide red. The powder was determined to be pure phase α-Fe by XRD 2 o 3 , The conversion rate is 99%. Observation by transmission electron microscope, iron oxide red product is spindle-shaped. The measured technical indicators of the...
Embodiment 2
[0023] Add 255g of chemical iron sludge (from Zhejiang Changshan Changsheng Chemical) into the water absorption tank of nitroxyl chloride, heat it with steam at 90-100°C, stir for 60 minutes to dissolve the iron sludge, and measure Fe in the solution 3+ After the content reaches 130g / L, filter. The filtrate is transferred to the sedimentation tank, and an appropriate amount of ammonia water is added to control the pH value to 6, so that all the ferric iron is formed into ferric hydroxide and precipitated, and the solid and liquid are separated by a centrifugal separator, and the solid ferric hydroxide is put into a heating kiln for heating at 110°C In 10 minutes, the solid turned red and decomposed to obtain 366g of pure iron oxide red. The powder was determined to be pure phase α-Fe by XRD 2 o 3 , The conversion rate is 99%. Observation by transmission electron microscope, iron oxide red product is spindle-shaped. The measured technical indicators of the product meet the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com