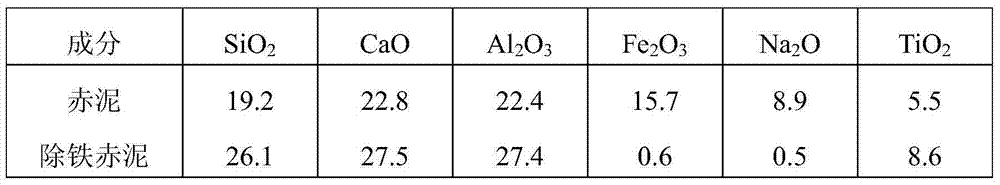
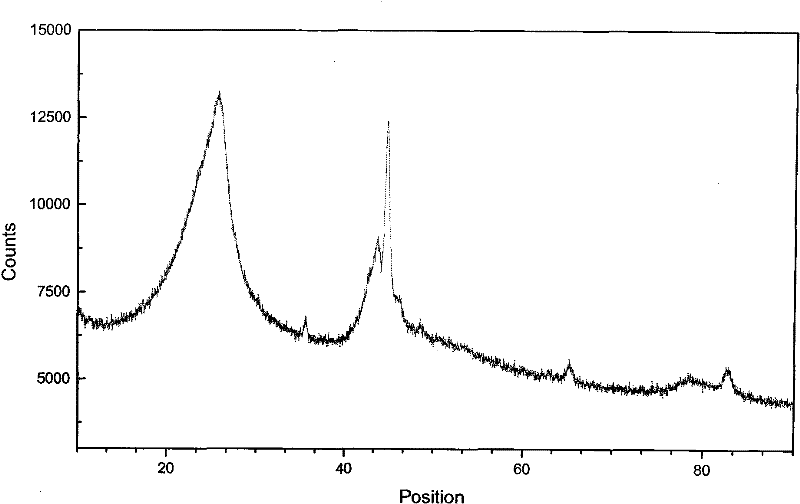
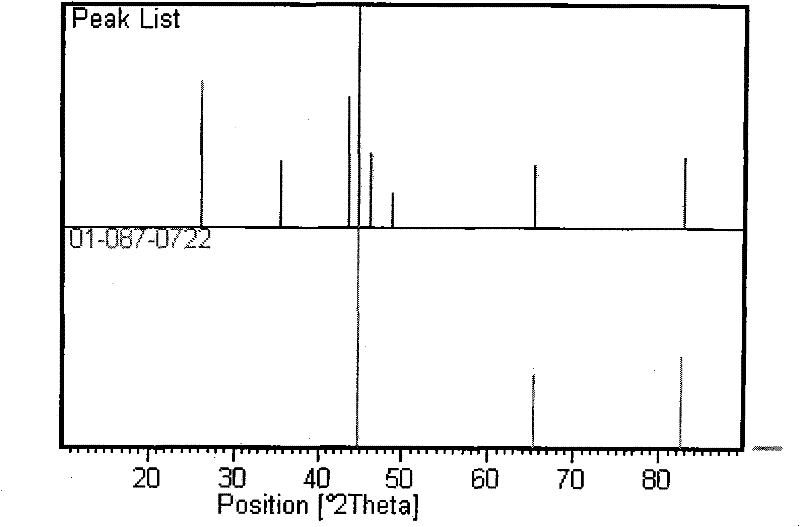
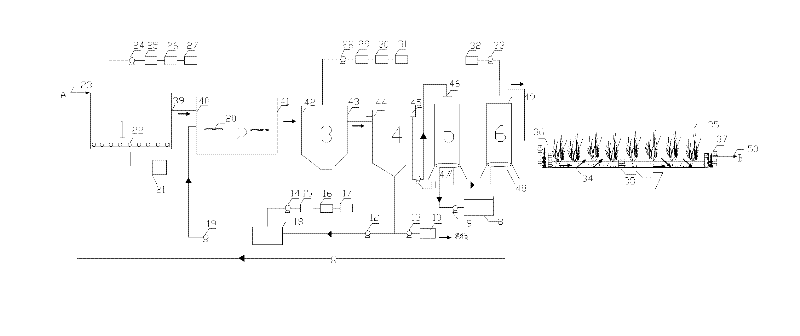
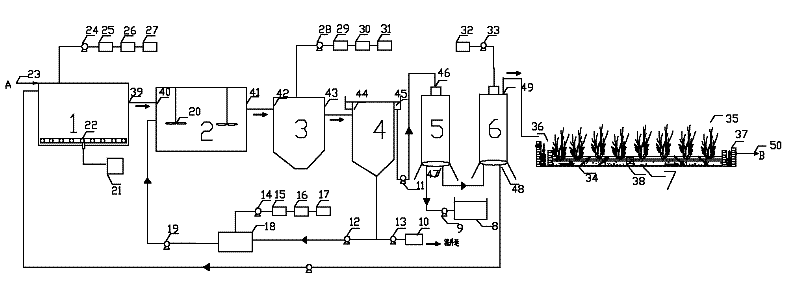
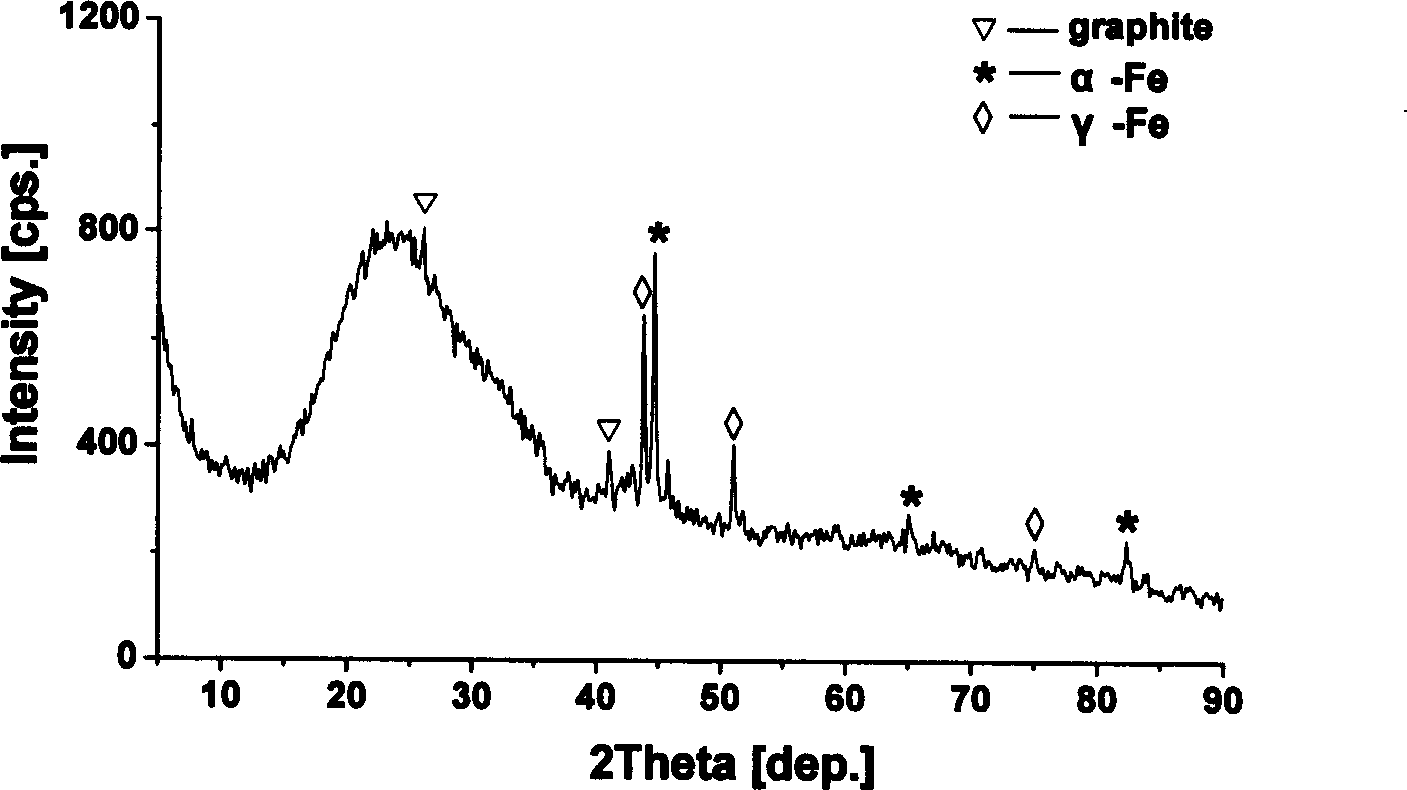
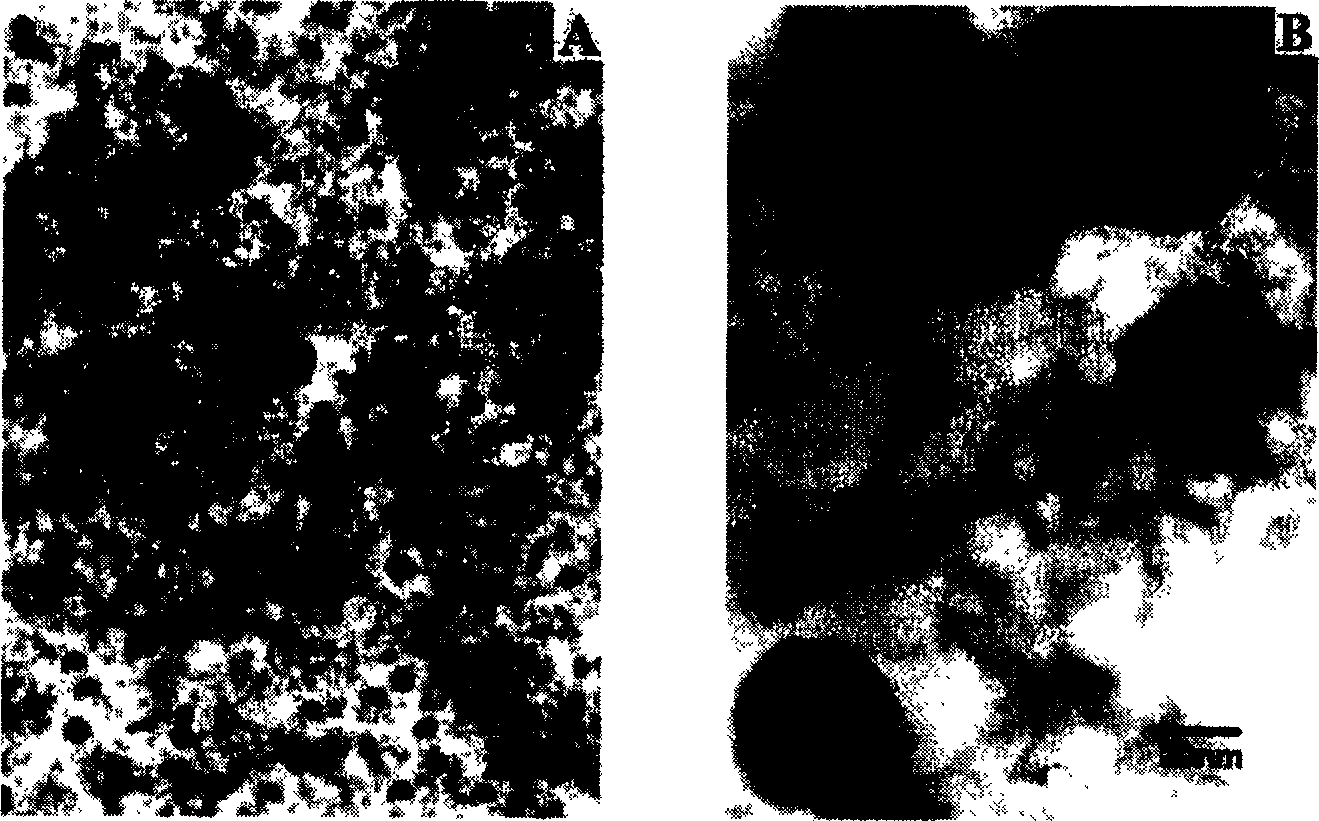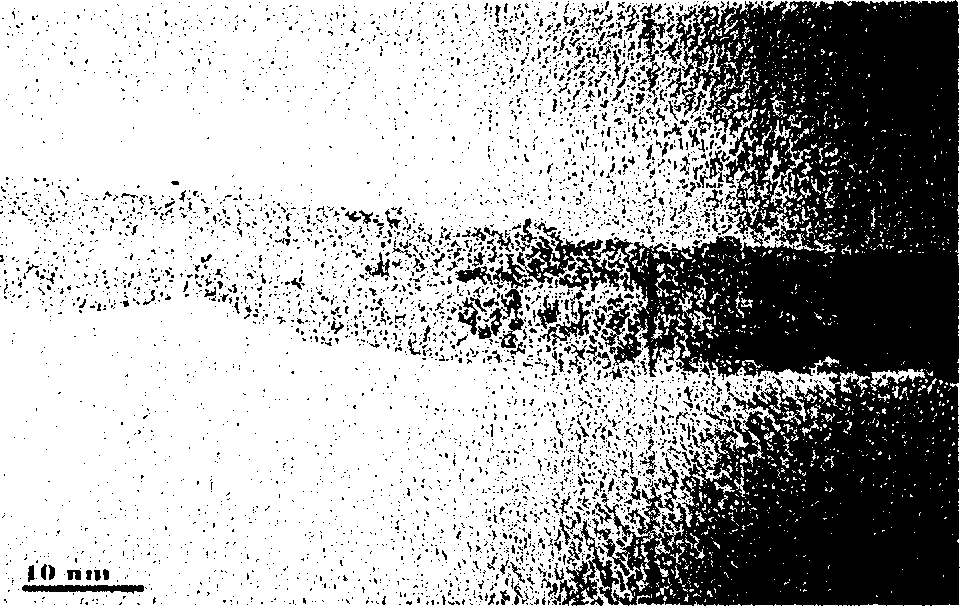Patents
Literature
131 results about "Ferric hydroxide oxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ferric hydroxide. A number of chemicals are dubbed iron(III) oxide-hydroxide. These chemicals are oxide-hydroxides of iron, and may occur in anhydrous (FeO(OH)) or hydrated (FeO(OH)·nHO) forms. The monohydrate (FeO(OH)·HO) might otherwise be described as iron(III) hydroxide (Fe(OH)), and is also known as hydrated iron oxide or yellow iron oxide.
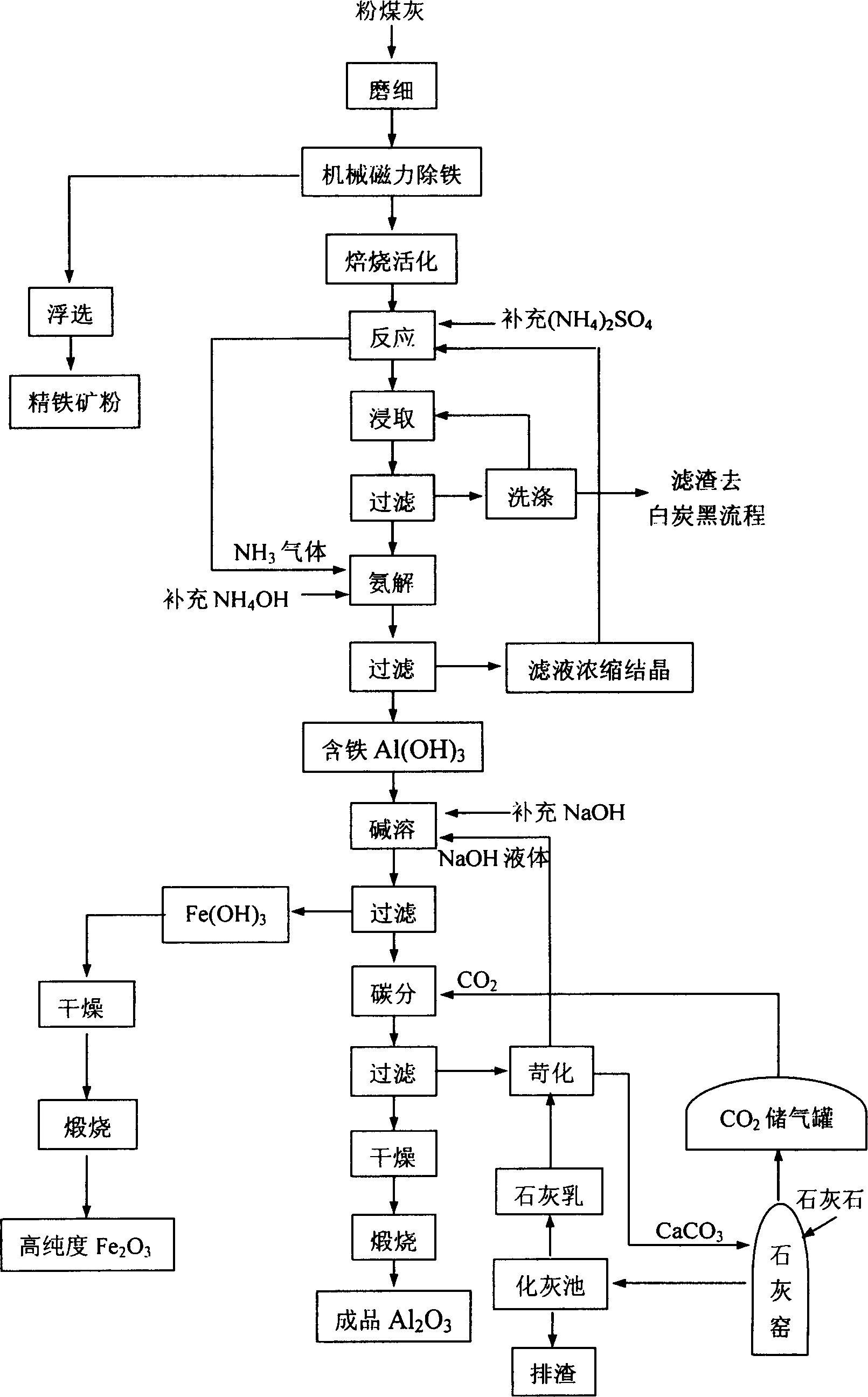
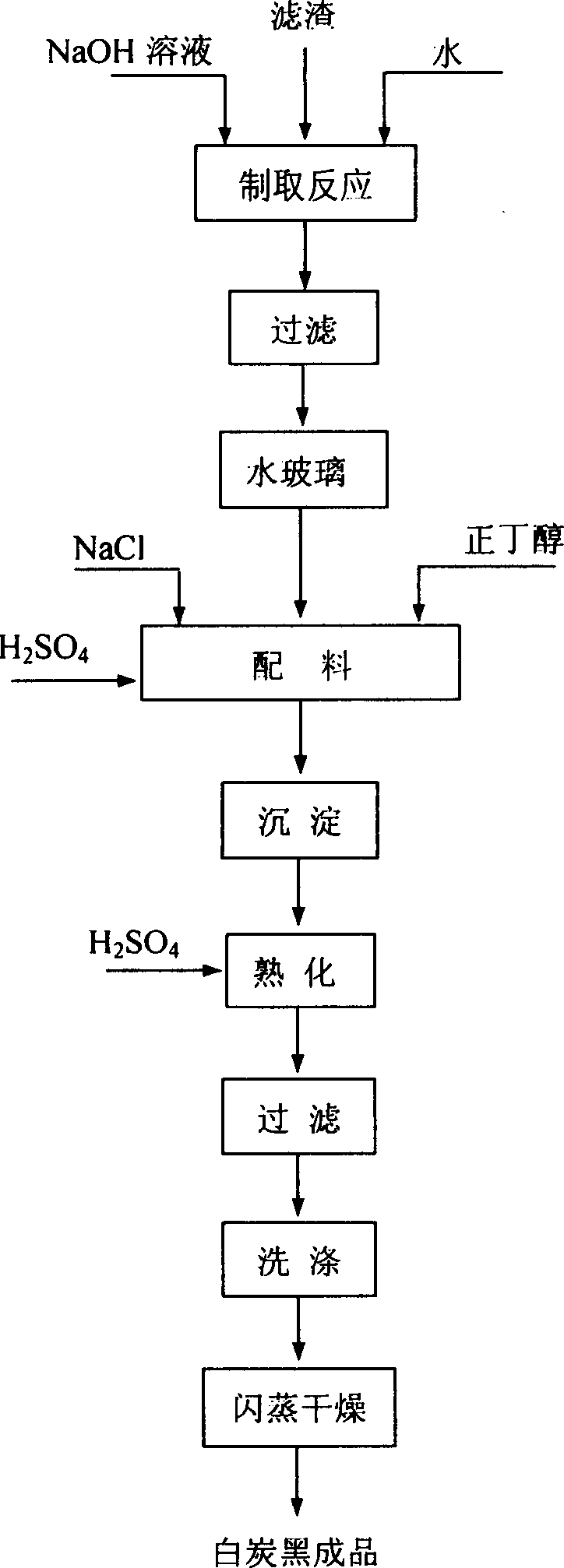
Method of extracting aluminium oxide from fly ash and simultaneously producing white carbon black
InactiveCN1868884AHigh activityUniform particle sizeSilicaAluminium oxides/hydroxidesAluminium hydroxideCoal
A process for extracting alumina from powdered coal ash while generating white carbon black includes such steps as grinding powdered coal ash, activating, adding ammonium sulfate, reacting, adding water, filtering, filling ammonia gas into the filtered liquid to obtain the deposited mixture of aluminum hydroxide and iron hydroxide, adding the solution of sodium hydroxide to dissolve the aluminum hydroxide deposit, adding carbon to obtain pure aluminum hydroxide, and calcining to obtain Al2O3.
Owner:NORTH UNITED POWER CO LTD
Multiple element composite metal oxidate arsenic removal settling agent and use method thereof
The invention belongs to the material field for removing the arsenic in water by settlement, more particularly relates to polyelement composite metal oxide arsenic removal settlement agent and an application method thereof based on iron oxide, manganese oxide, and aluminum oxide. The invention uses an ectopic preparation method or situ preparation method for preparing and obtaining polyelement composite metal oxide arsenic removal settlement agent which comprises hydrated iron oxide, hydroxyl-hydrated ferric hydroxide, hydrated aluminum oxide, hydroxyl-hydrated alumina, hydrated manganese oxide and the like. The polyelement composite metal oxide arsenic removal settlement agent can be used for removing the arsenic pollutants in water bodies such as lakes, reservoirs, rivers, groundwater, drinking water, industrial waste water, and the like; in addition, the settlement agent can also be used for removing the heavy metals such as copper, chromium, cadmium, lead, and the like, and the pollutants such as iron, manganese, phosphates, and the like in water by settlement.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Textile composite with iron oxide film
A colored textile composite is produced by forming an iron (III) oxide film on a textile surface. This is accomplished by contacting the textile with an aqueous solution having an iron (II) or iron (III) species present. The iron (II) ion resulting from the dissociated iron (II) salt, if an iron (II) salt is utilized, is first hydrolyzed within the aqueous solution and then oxidized under controlled conditions to form iron (III) oxide (hydroxide). The iron (III) ion resulting from the dissociated iron (III) salt, if an iron (III) salt is utilized, is only hydrolyzed under controlled condition to form iron (III) oxide (hydroxide). The iron (III) oxide is then nucleated and forms a smooth and coherent iron (III) oxide film or coating on the surface of the textile without forming an insoluble iron (III) hydroxide precipitate in the solution. This reaction occurs because the reaction conditions are controlled in such a manner as to form sub-colloidal sized iron oxide particles which, in turn, permits a faster rate of adsorption of the iron (III) oxides onto the substrate surface than the rate of formation of the same particles. The iron (III) oxide formed may be goethite, hematite, or magnetite or any mixture thereof. Varying the type of oxide formed allows control over the color shade and other properties of the treated textile composite.
Owner:MILLIKEN & CO
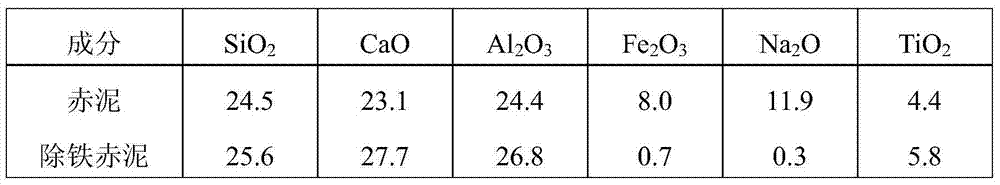
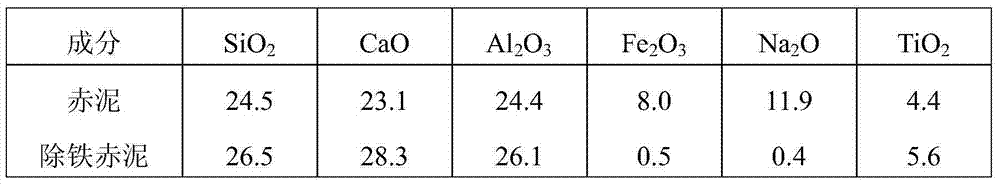
Method for separating and recovering iron from red mud
The invention relates to a method for separating and recovering iron from red mud. The method is characterized by comprising the steps: of leaching out and removing iron from the red mud by using an oxalic acid solution, and filtering to obtain iron-free red mud and an iron oxalate-containing leaching solution, wherein the obtained iron-free red mud can be used as a raw material for producing cement and fireproof bricks; iron oxalate in the obtained leaching solution is reduced to ferrous oxalate through membrane electrolysis or adding a reducing agent and ferrous oxalate is precipitated out, or iron in the solution is separated by directly neutralizing and precipitating an iron hydroxide and oxalate mixture; obtained ferrous oxalate is decomposed to obtain oxalic acid and an iron-containing compound; the obtained iron hydroxide and oxalate mixture is selectively leached out, separated and recovered to obtain oxalic acid and an iron-containing compound; the process of returning recovered oxalic acid to the red mud to leach out and remove iron is cyclically used. The method has the characteristics of short technological process, good iron separation effect, simplicity and convenience in operation, environmental friendliness and the like, and is suitable for large-scale industrialized application.
Owner:CENT SOUTH UNIV
Novel method for preparing load nano zero valent iron
InactiveCN102205419AUniform size distributionPrevent restoreMaterial nanotechnologyCoalReducing agent
The invention discloses a novel method for preparing load nano zero valent iron. The iron source is iron-containing compounds of Fe<2+> and Fe<3+>, such as ferric oleate, ferric hydroxide oxide, ferric chloride, ferric nitrate and the like; and the loads are carbon black, active carbon, kieselguhr, zeolite and other load materials. The method is characterized in that: the iron-containing compounds on the loads are reduced into nano zero valent iron under the atmosphere condition and under the reduction action of reducing agents, such as H2, CO, and reduction carbon (such as coke, reduction coal and the like). The preparation method is environment-friendly, and simple and is low in cost; the expensive reducing agents (such as NaBH4 and the like) are avoided, and organic pollutants (such as halogenated hydrocarbon, pesticide, polychlorinated biphenyls (PCBs) and polycyclic aromatic hydrocarbons (PAHs), inorganic negative ions (such as NO<3->), and heavy metal (such as Cr<6+>, As<3+>, As<5+>, Cu<2+>, Pb<2+>) and the like can be efficiently removed effectively.
Owner:BEIJING NORMAL UNIVERSITY
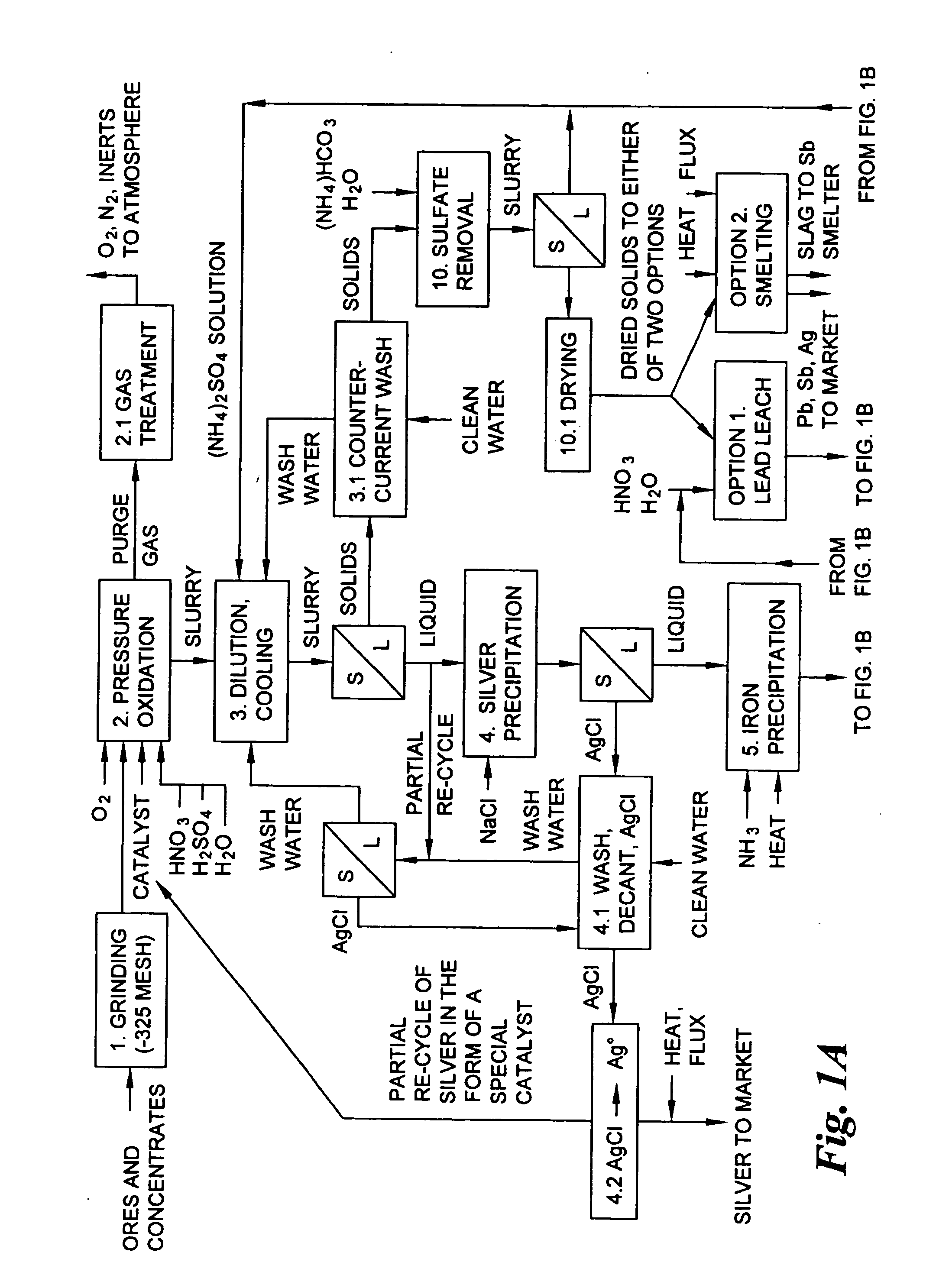
Hydrometallurgical process for the treatment of metal-bearing sulfide mineral concentrates
InactiveUS20070098609A1Improve efficiencyReduce the amount requiredSolvent extractionGold compoundsAmmonium compoundsMetallic sulfide
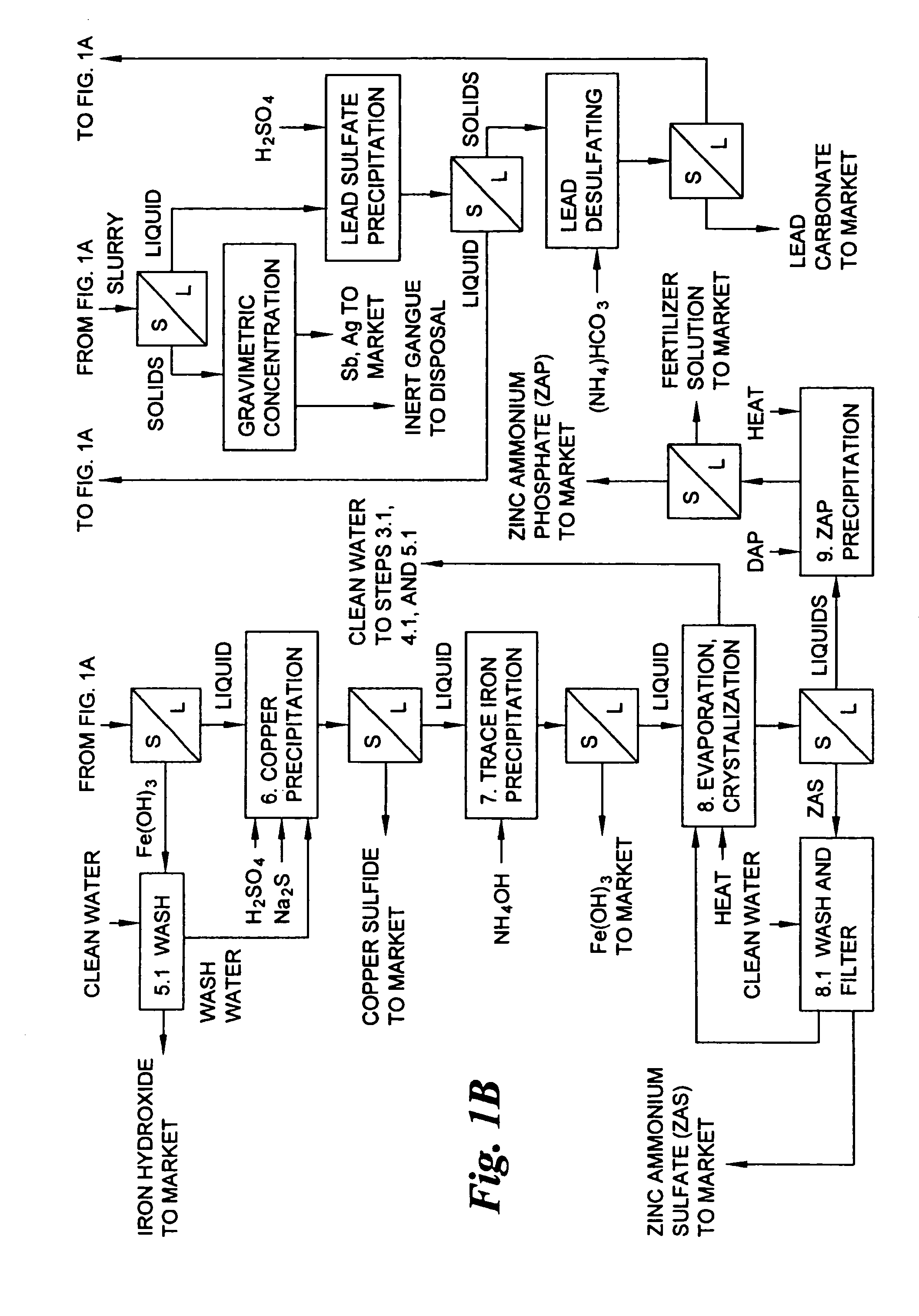
A hydrometallurgical process for the treatment of complex silver-bearing sulfide ores and concentrates that recovers substantially all silver, lead, antimony, zinc, copper and sulfur, along with the chemical reagents utilized during the process. Finely ground ores and concentrates are leached under heat and pressure with water, sulfuric acid, nitric acid, oxygen, and a catalyst, and are further treated to recover silver in the form of silver chloride; iron in the form of iron hydroxide; copper and all traces of soluble toxic metals as sulfides; zinc as zinc ammonium sulfate and specifically nitric acid, sulfuric acid, oxygen, ammonia, and ammonium compounds as valuable fertilizer products.
Owner:ROYAL SILVER PANAMA
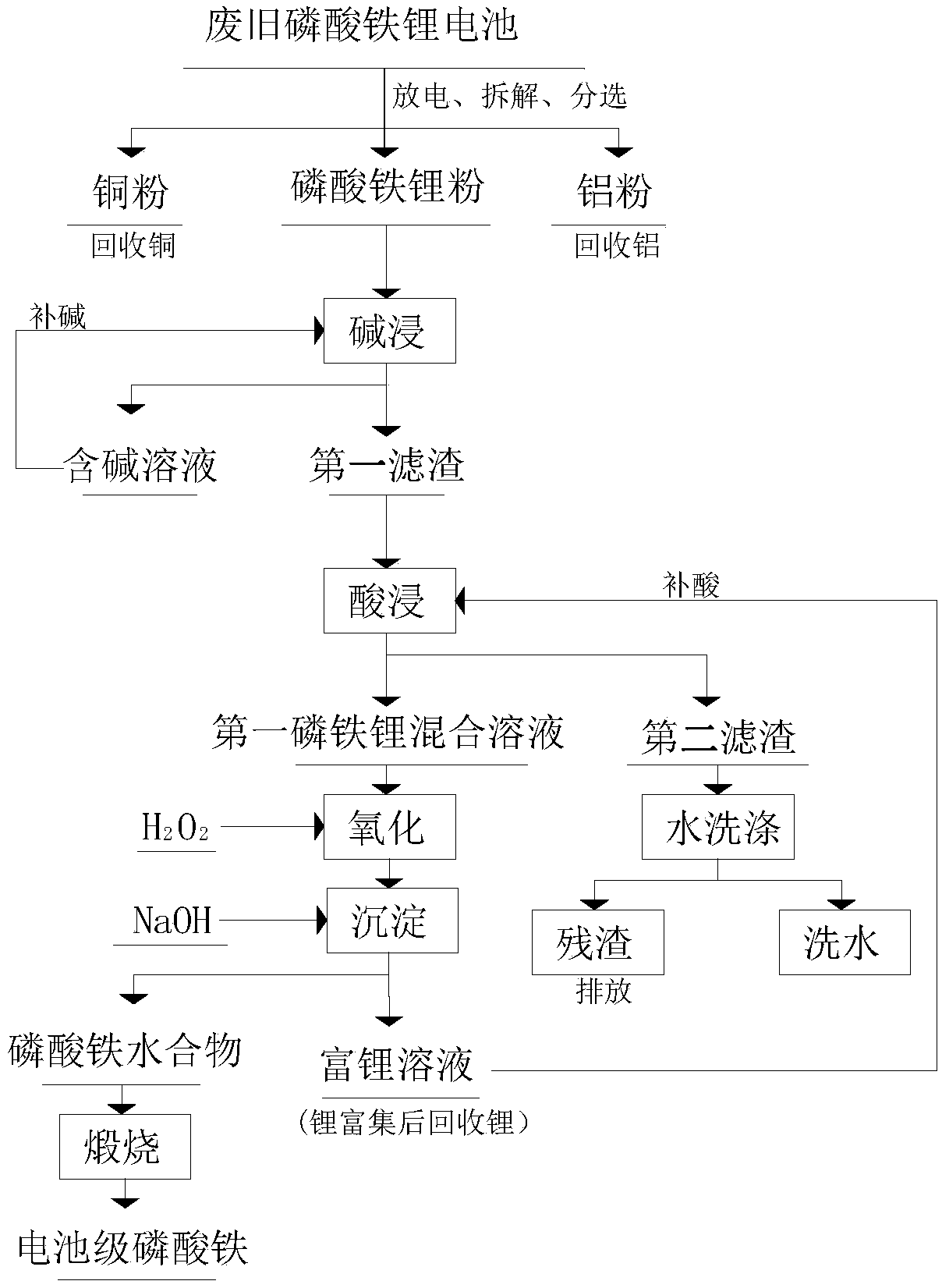
Method for preparing battery grade iron phosphate from waste and old lithium iron phosphate batteries
ActiveCN109179358AEfficient removalHigh purityPhosphorus compoundsLithium iron phosphatePhosphoric acid
The invention discloses a method for preparing battery grade iron phosphate from waste and old lithium iron phosphate batteries and relates to the technical field of battery recovery. The method can prepare anhydrous battery grade iron phosphate by battery disassembly and separation, alkali leaching, acid leaching, oxidation, precipitation and calcination. Through alkali leaching and then acid leaching and precipitation, impurities such as aluminum in lithium iron phosphate can be removed and the purity of iron phosphate can be improved. The alkali leaching solution and the lithium-rich solution can be reused so that a recovery cost is reduced. After repeated acid supply, the lithium-rich solution immerses novel filter wastes so that the concentration of lithium in the solution and the recovery rate of lithium can be increased and the recovery cost of lithium can be reduced. pH at the precipitation end point is low in a range of 1.0-2.5 so that the formation trend of iron hydroxide isreduced. The aging process after the precipitation reaction can improve the purity of iron phosphate and the produced iron phosphate meets the industry standards. The whole process is carried out at alow temperature so that the corrosion of the solution to the equipment is delayed and the energy consumption and recycling cost are reduced.
Owner:INST OF RESOURCES UTILIZATION & RARE EARTH DEV GUANGDONG ACAD OF SCI
Process and method for the removal of arsenic from water
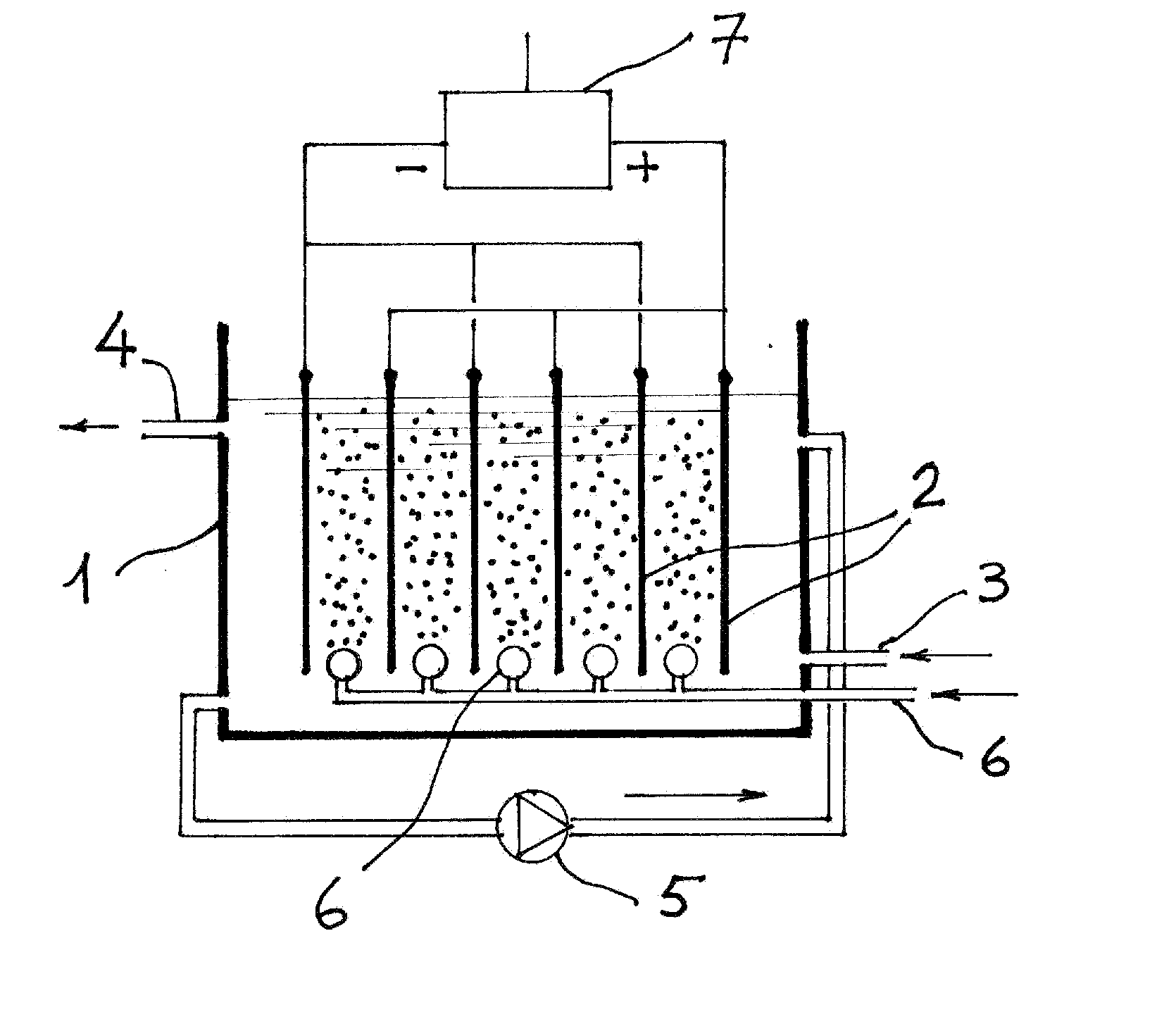
This invention describes a one step process for the removal of heavy metals, particularly arsenic, from water. The process consists in promoting the circulation of the water to be treated in an electrolytic cell equipped with iron, or iron alloy anodes and cathodes made of iron or iron alloy or other metals, while the contemporary insufflation into the cell of a gas, partially or totally composed of oxygen. In this way the iron of the anode electrodes dissolves as iron hydroxide. The ferrous hydroxide thus generated, under the action of the oxygen contained in the insufflated gas is converted to ferric hydroxide, which, through a complex mechanism, adsorbs and forms insoluble complexes with the arsenic ions. At the same time As(III) is subject to oxidation both at the anode and at the cathode. By this process both forms of arsenic, As(III) and As(V), are equally removed. The treated water is further processed by conventional clarifying and filtering.
Owner:DEL SIGNORE GIOVANNI
Iron Precipitation
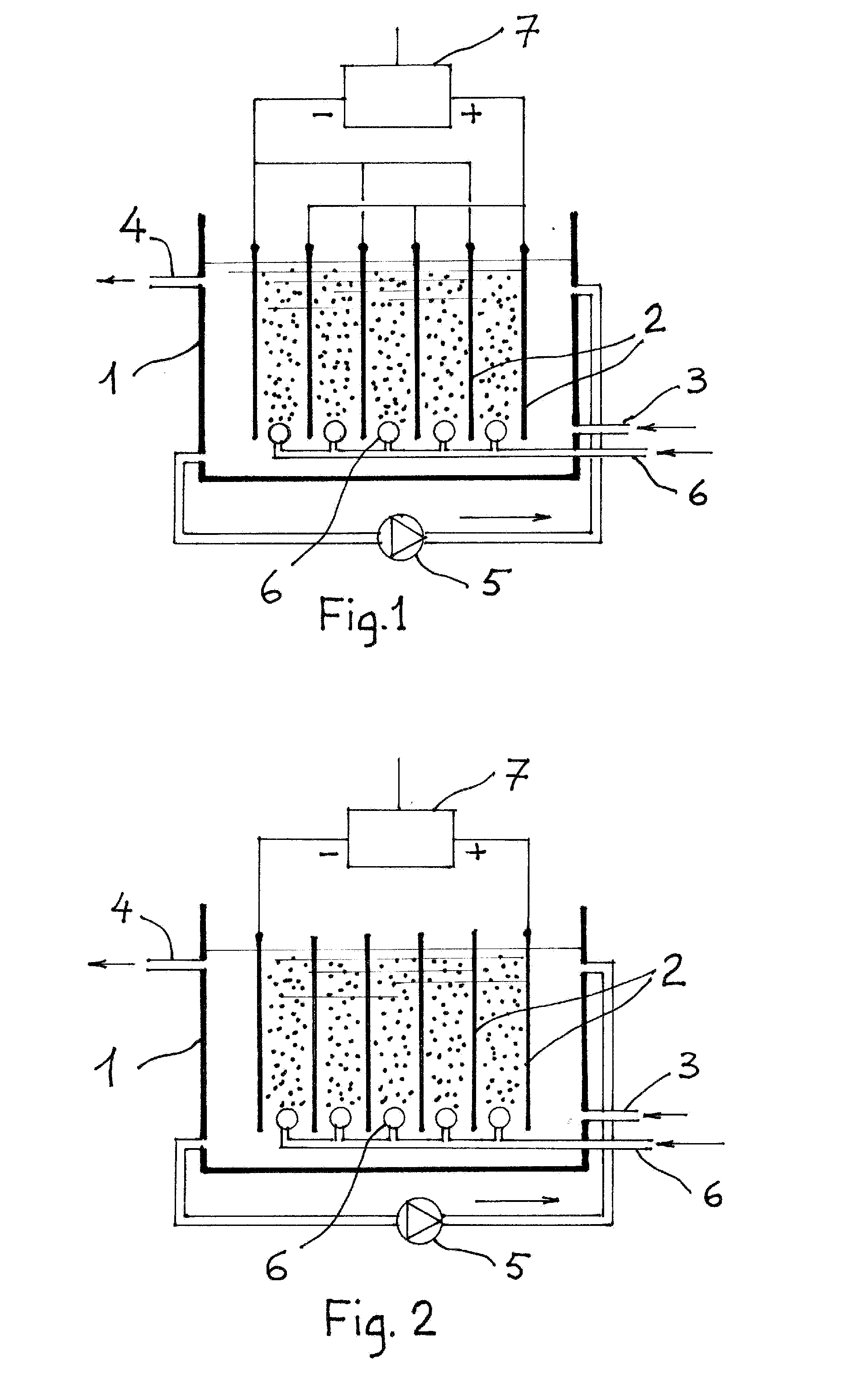
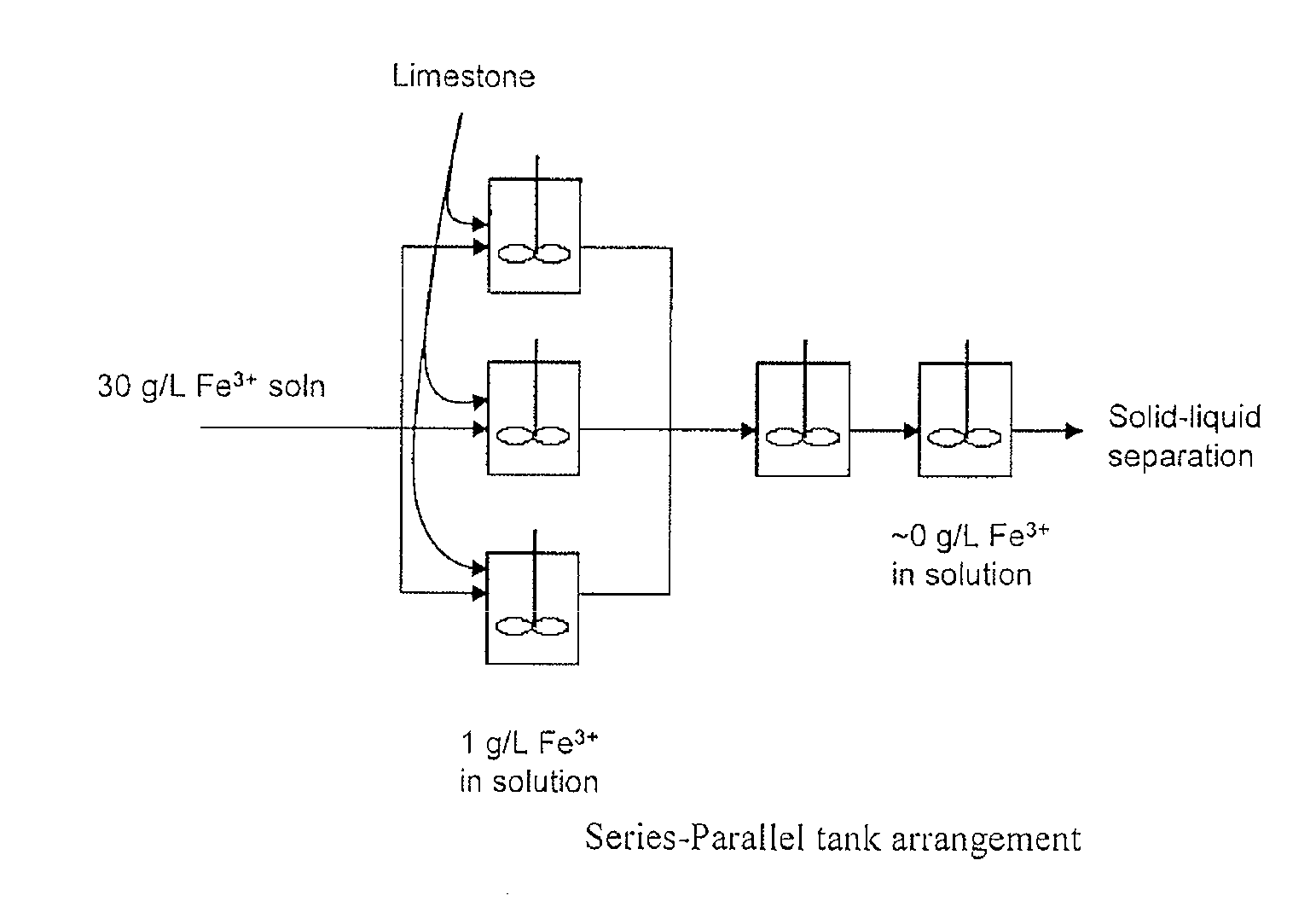
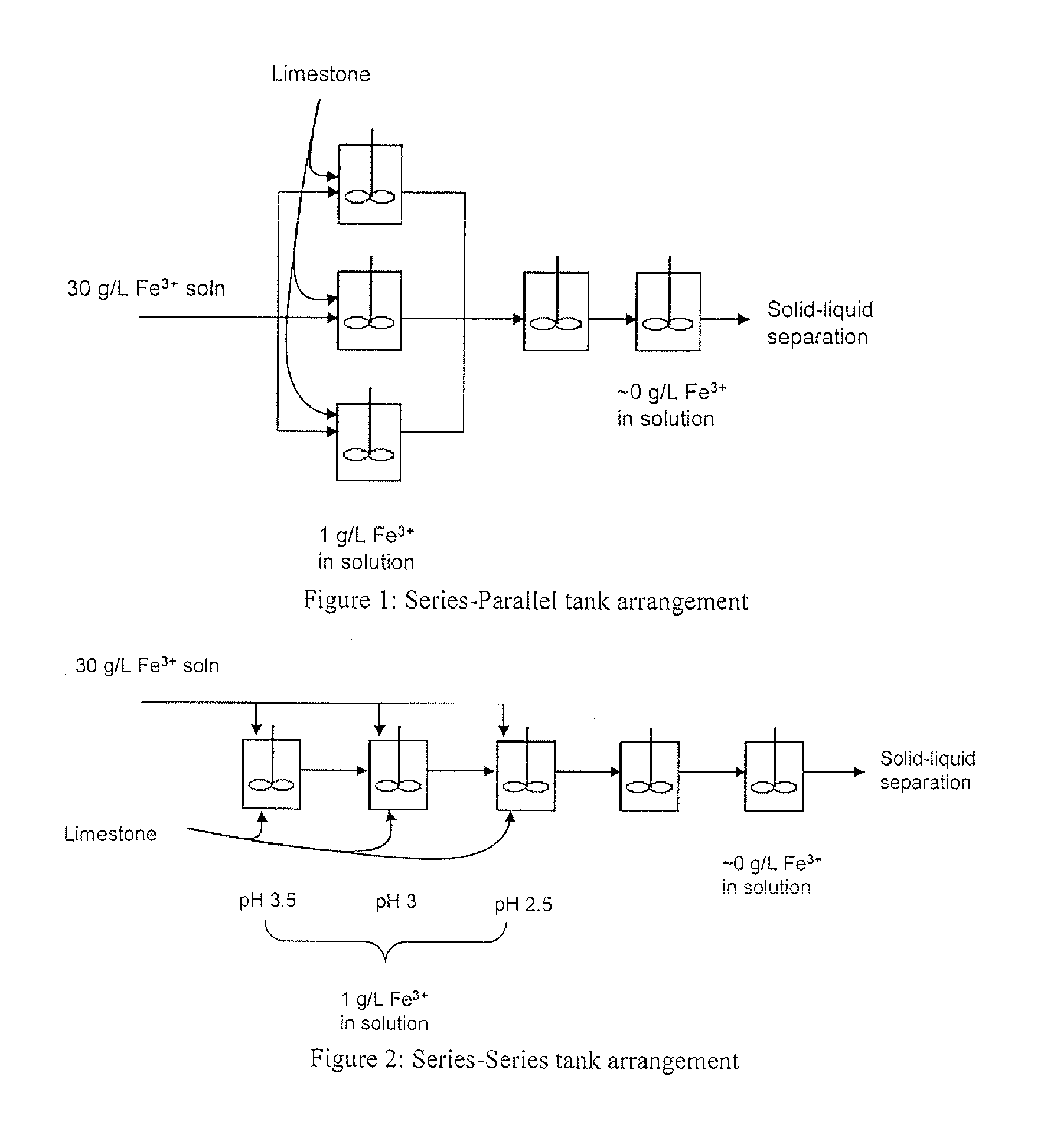
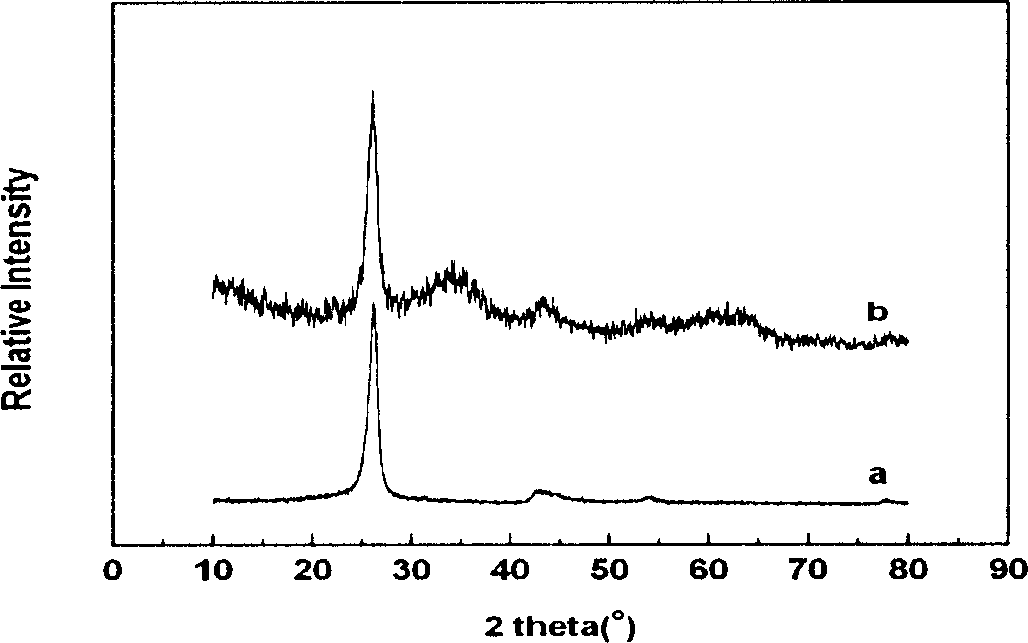
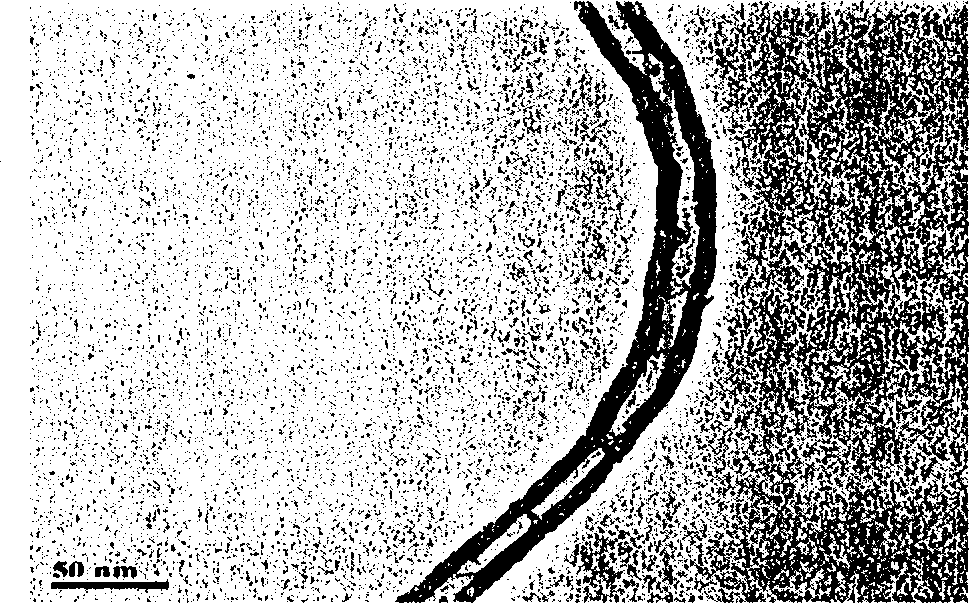
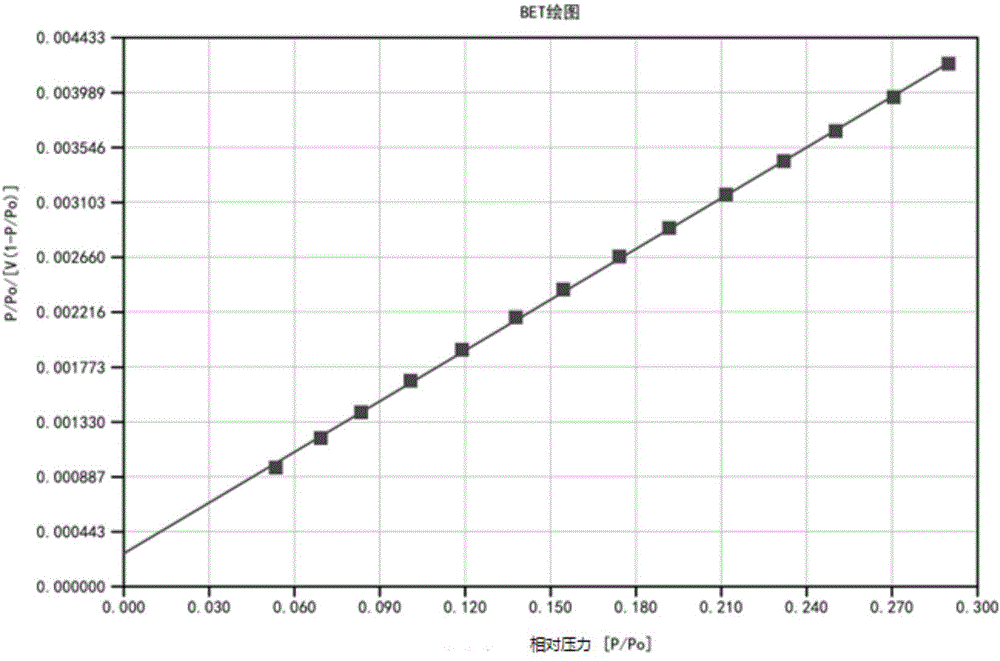
InactiveUS20110120267A1Reduce lossesIncreased formationSolvent extractionIron compoundsFerric hydroxide oxideResidence time
A process for the treatment of a solution containing at least ferric ions, and one or more metal values, said process including the step of maintaining a controlled concentration of ferric ions in solution for a sufficient residence time to control iron hydroxide or oxide crystal growth, and precipitating the iron as a relatively crystalline iron hydroxide or oxide while minimising the loss of the ore or more metal values with the iron hydroxide or oxide.
Owner:BHP BILLITON SSM TECH PTY LTD
Method for removing trace of thallium in sewage
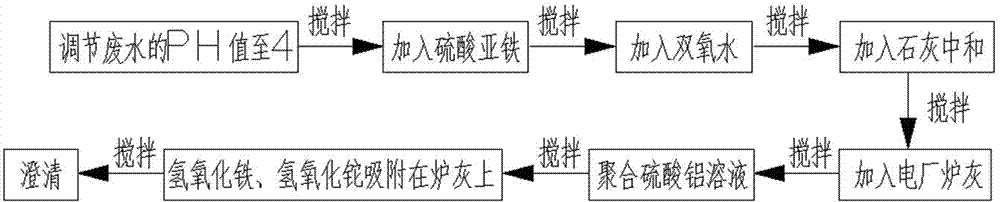
InactiveCN104773878AStrong oxidizingImprove adsorption capacityWater contaminantsMultistage water/sewage treatmentFenton reagentSulfate
The invention relates to a method for removing a trace of thallium in sewage. The method includes the process steps of regulating the PH value of the thallium-contained sewage to 4, adding a ferrous sulfate solution to the sewage, evenly mixing the sewage with the ferrous sulfate solution, adding hydrogen peroxide to the sewage again, conducting stirring, oxidizing fluorosis univalence thallium (T1+) in the sewage into tervalence thallium (T13+), mixing ferrous iron and hydrogen peroxide into a fenton reagent, adding lime to the sewage and evenly conducting stirring so that the PH value of the sewage can be neutralized to be 7 to 9, hydrolyzing tervalence iron (Fe3+) in the sewage at the same time to form flocculent ferric hydroxide, adding an appropriate amount of power plant stove ash (electric duct collector stove ash) to the sewage, evenly conducting stirring, and adding a polyaluminium sulfate solution so that ferric hydroxide and thallium hydroxide can be adsorbed to the stove ash. Flocculent sediment generated through hydrolysis has the great surface area and quite strong adsorption capacity; the power plant stove ash can adsorb the flocculent sediment and colloid particles in the sewage and sink.
Owner:广东云测环境科技有限公司
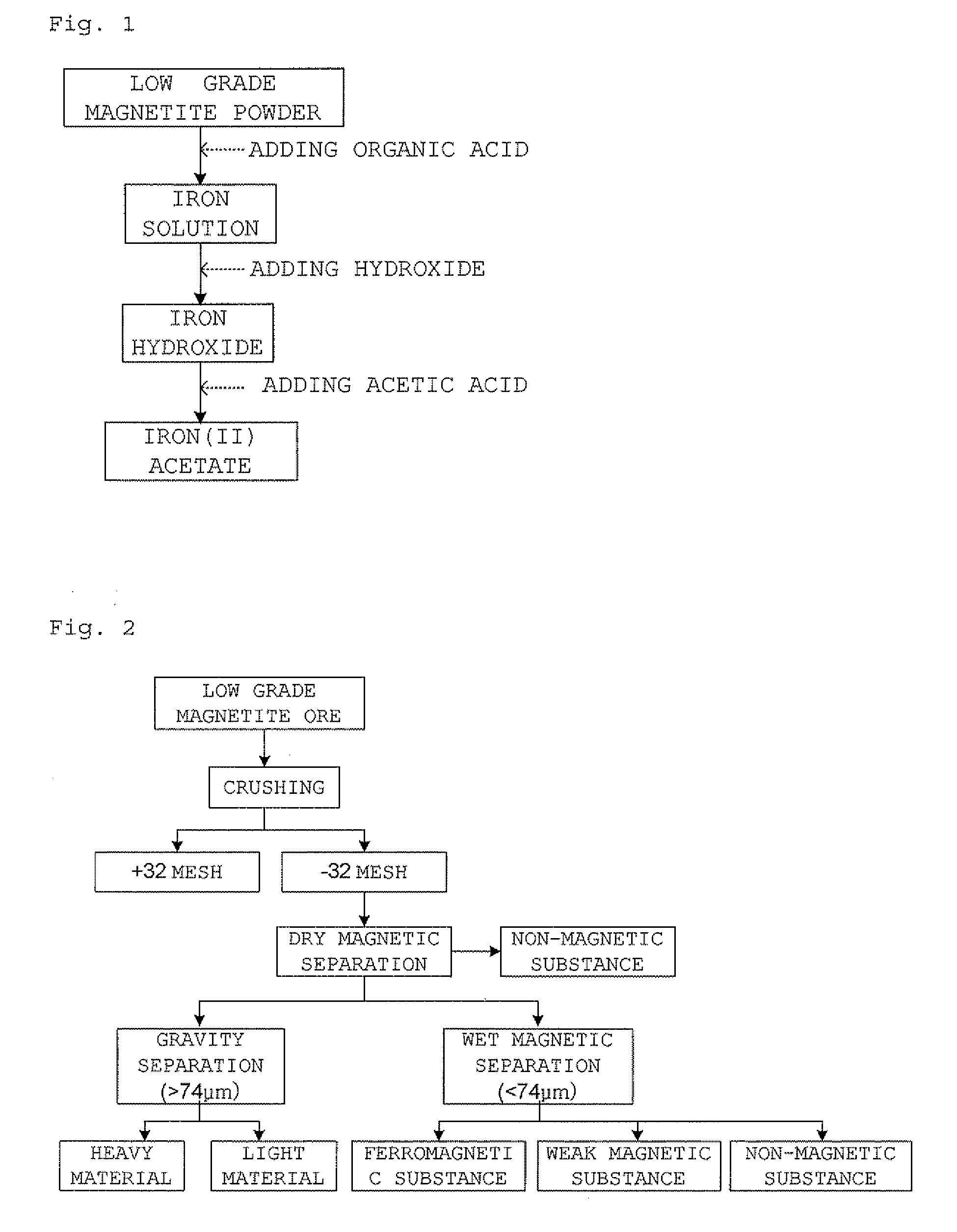
Preparation of iron (II) acetate powder from a low grade magnetite
InactiveUS20090105493A1High yieldShort reaction timeOrganic compound preparationIron organic compoundsAcetic acidOrganic acid
The present invention relates to a preparation of iron(II) acetate powder from low grade magnetite and comprises the following steps: (a) adding organic acid to low grade magnetite powder to obtain iron solution; (b) adding hydroxide to the iron solution to obtain iron hydroxide; and (c) adding acetic acid to the iron hydroxide, thereby obtaining iron(II) acetate.According to the present invention, it is possible to obtain high purity iron(II) acetate using low grade magnetite and there are advantages of mass producible environmentally-friendly simple process and prevention of corrosion of facilities.
Owner:KOREA INST OF GEOSCI & MINERAL RESOURCES
Method for treating oil field drilling waste water
ActiveCN102030435AImprove biodegradabilityImprove surface activityMultistage water/sewage treatmentWater/sewage treatment by flocculation/precipitationChemical oxygen demandPolyacrylamide
The invention relates to a method for treating oil field drilling waste water, which comprises the following major steps: A) adding activated carbon and ferric trichloride, ferric sulfate or other ferric salt to oil field drilling waste water, and introducing ozone to perform a catalytic ozonization reaction; and B) after ozonization, adding polyacrylamide flocculant to the outlet water to enablethe activated carbon and ferric hydroxide to be subject to coagulative precipitation, and separating the water and precipitates. By using the method provided by the invention, the COD (Chemical Oxygen Demand) (2000-10000mg / L) removal rate of the oil field drilling waste water can be increased from 10% or so to 50-70% by ozone oxidation.
Owner:北京中科国益环保工程有限公司
Organic-inorganic ionic hetero high efficiency flocculant
InactiveCN1554592AIncrease profitReduce dosageWater/sewage treatment by flocculation/precipitationMicroparticleOrganic polymer
The efficient flocculant consists of hydroxide colloid 0.5-15 wt%, acrylamide 50-95 wt% and cationic monomer 0-45 wt%. The hydroxide colloid may be aluminum other, iron hydroxide, magnesium hydroxide or zinc other, and has colloid size of 10-200 nm. The cationic monomer includes DMDAAC or DMC. The organic-inorganic hetero efficient flocculant has in-situ polymerized acrylamide / cationic monomer copolymer linked to the surface of charged inorganic colloid particle, so that it has the synergistic effect of electrically neutralizing effect of inorganic flocculant and the bridging effect of organic polymer and greatly raised flocculating efficiency.
Owner:ZHEJIANG UNIV
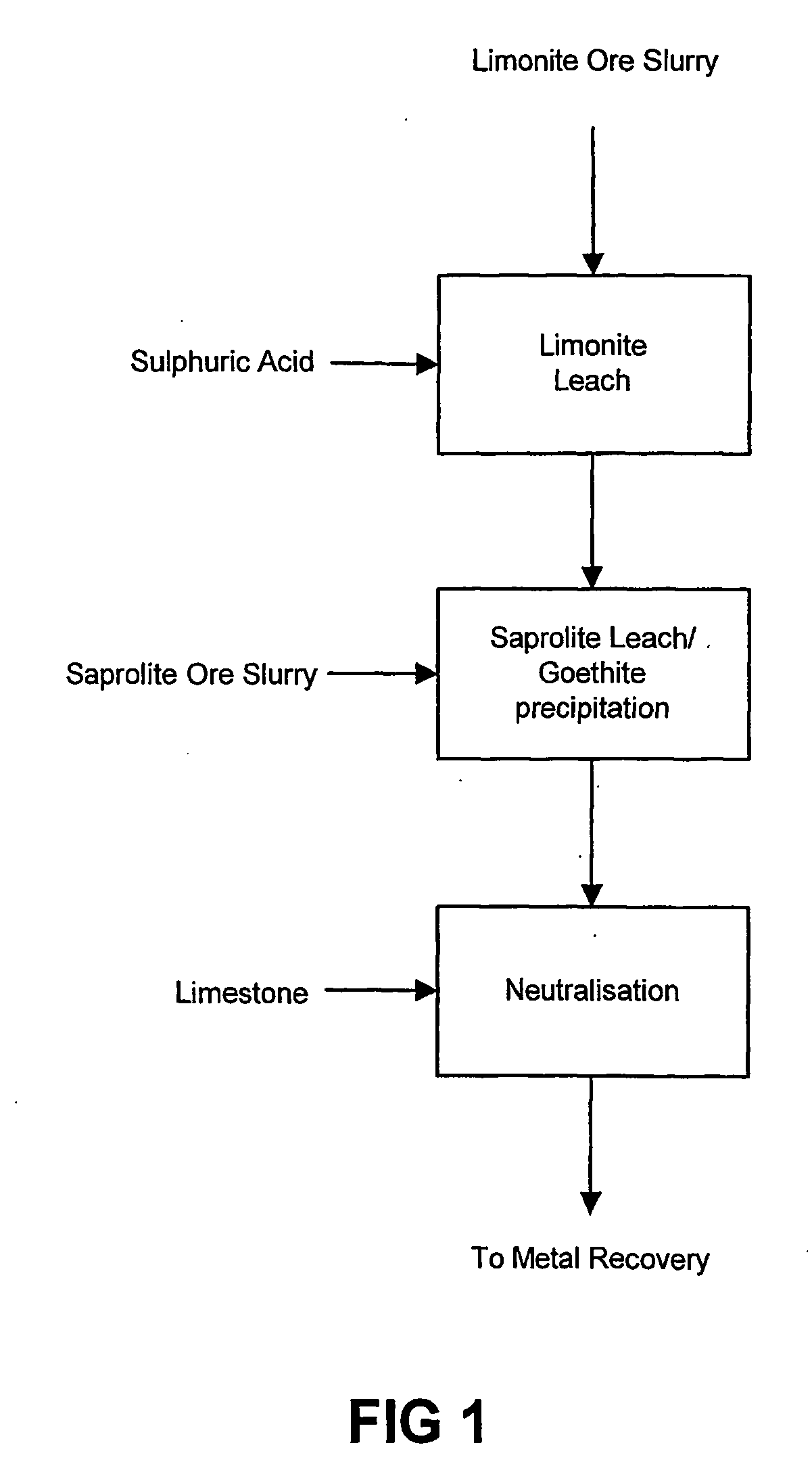
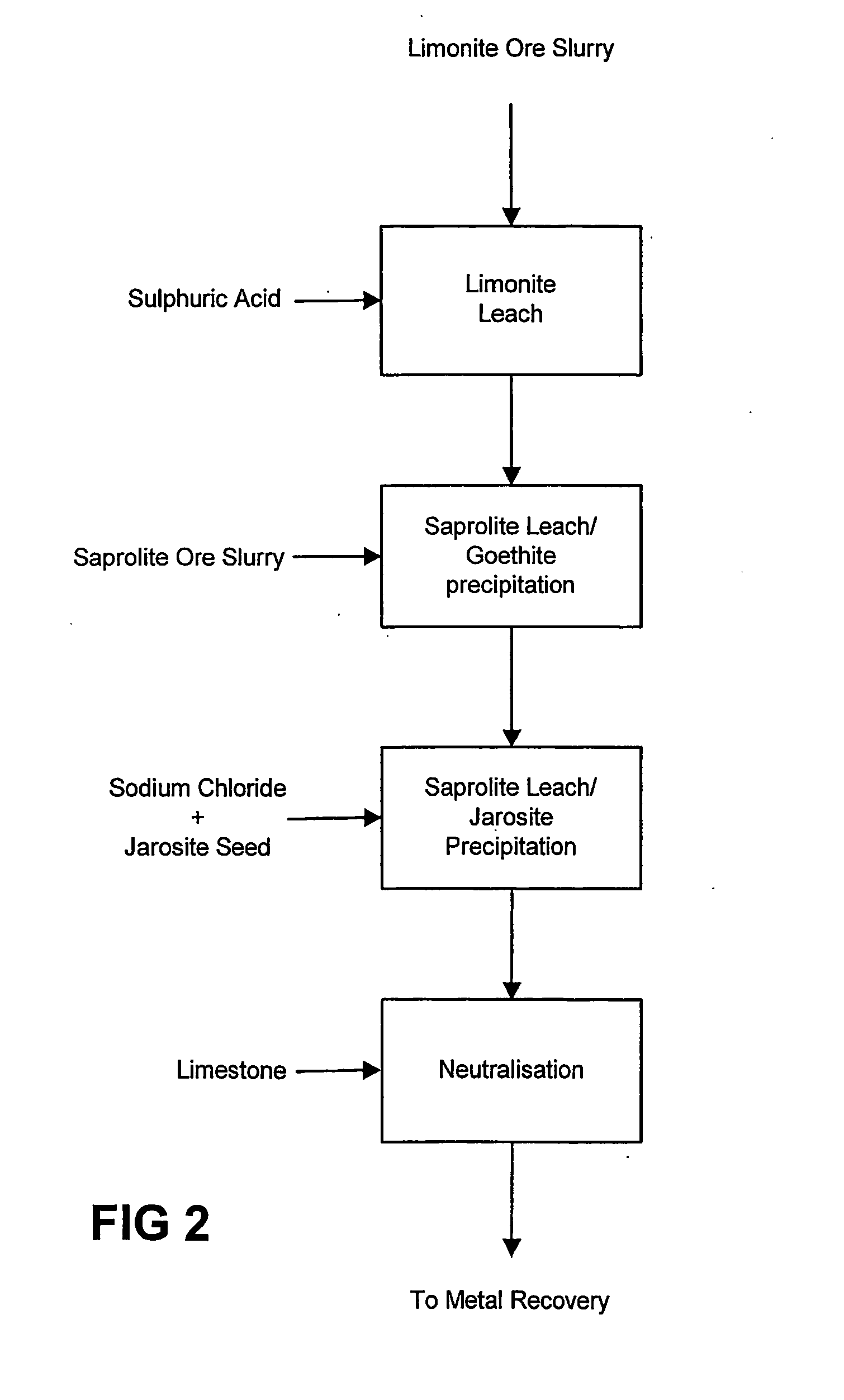
Atmospheric pressure leach process for lateritic nickel ore
An atmospheric leach process in the recovery of nickel and cobalt from lateritic ores, said processing including the steps of: a) separating the lateritic ore into a low magnesium containing ore fraction, and a high magnesium containing ore fraction by selective mining or post mining classification; b) separately slurrying the separated ore fractions; c) leaching the low magnesium containing ore fraction with concentrated sulphuric acid as a primary leach step; and d) introducing the high magnesium content ore slurry following substantial completion of the primary leach step and precipitating iron as goethite or another low sulphate containing form of iron oxide or iron hydroxide, wherein sulphuric acid released during iron precipitation is used to leach the high magnesium ore fraction as a secondary leach step.
Owner:CERRO MATOSO
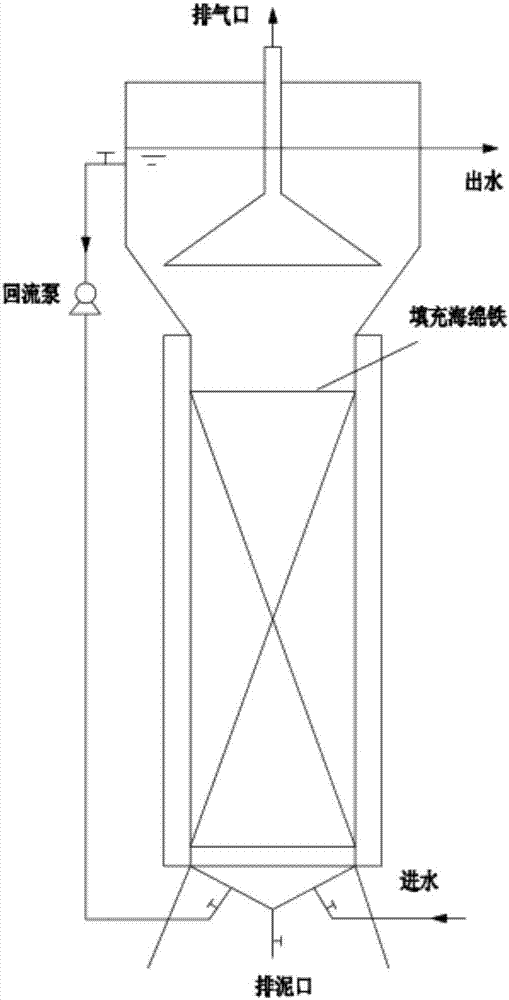
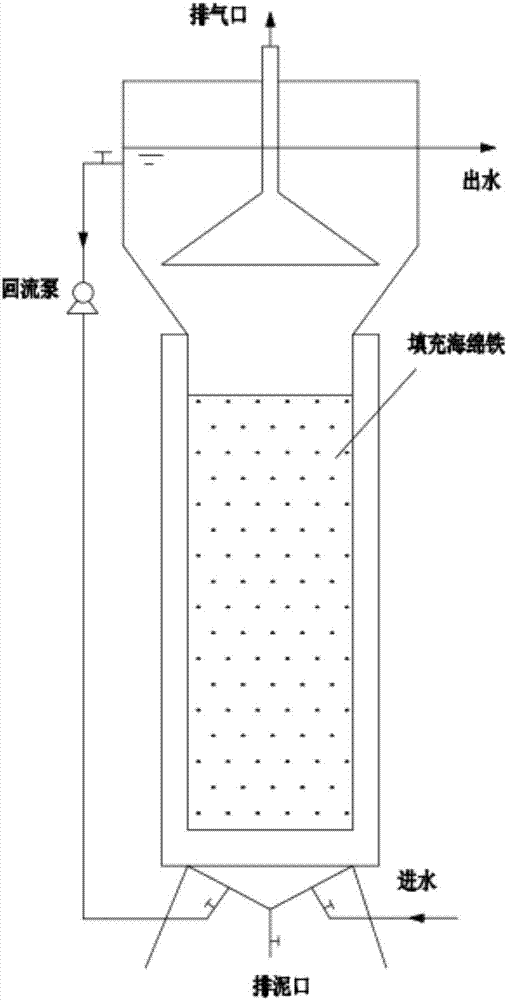
A process of reinforcing the denitrification efficiency of an anaerobic ammoxidation reactor by utilizing a spongy iron filler
InactiveCN106865760AEliminate the effects ofPromote growthWater contaminantsTreatment with anaerobic digestion processesSludgeTotal nitrogen
A process of reinforcing the denitrification efficiency of an anaerobic ammoxidation reactor by utilizing a spongy iron filler is provided. The process is characterized in that spongy iron is added into the reactor to increase the anaerobic ammoxidation denitrification efficiency. Through reduction, the spongy iron can remove dissolved oxygen or other oxidizing compounds possibly brought by raw water, and can eliminate inhibiting functions of the dissolved oxygen and the oxidizing compounds on anaerobic ammonium oxidation bacteria. The spongy iron plays a role on conversion and removal among ammonia nitrogen, nitrate nitrogen and nitrite nitrogen, thus reducing the total nitrogen in discharged water. Divalent ferrous ions and ferric ions which are dissolved out through a reaction from the spongy iron can be adopted as trace elements and supplied to microorganisms to promote physiological and biochemical reactions of the microorganisms, and generated ferric hydroxide floc can be adopted as a flocculating agent to promote formation of anaerobic granular sludge, thus reinforcing ammoxidation and increasing the removing rate of the total nitrogen. The process can be used for denitrification of domestic wastewater and can be used for denitrification of various types of nitrogen-containing industrial wastewater.
Owner:NORTHEAST DIANLI UNIVERSITY
Reducing water purification material, method for producing reducing water purification material, method for treating wastewater, and wastewater treatment apparatus
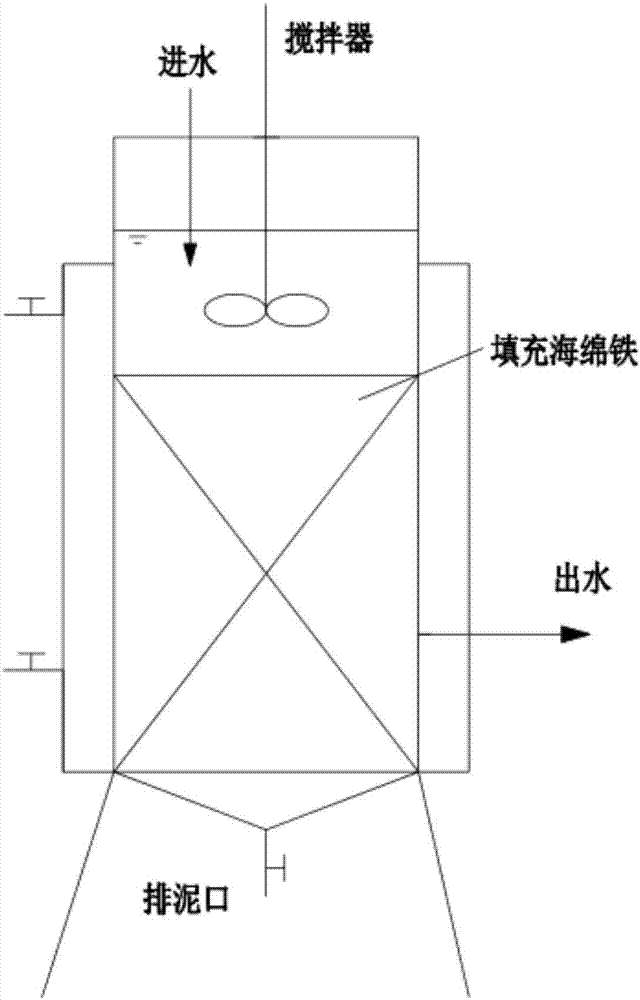
ActiveUS20070209989A1Satisfactory separationImprove economyLiquid degasificationWater softeningSludgeFerric hydroxide oxide
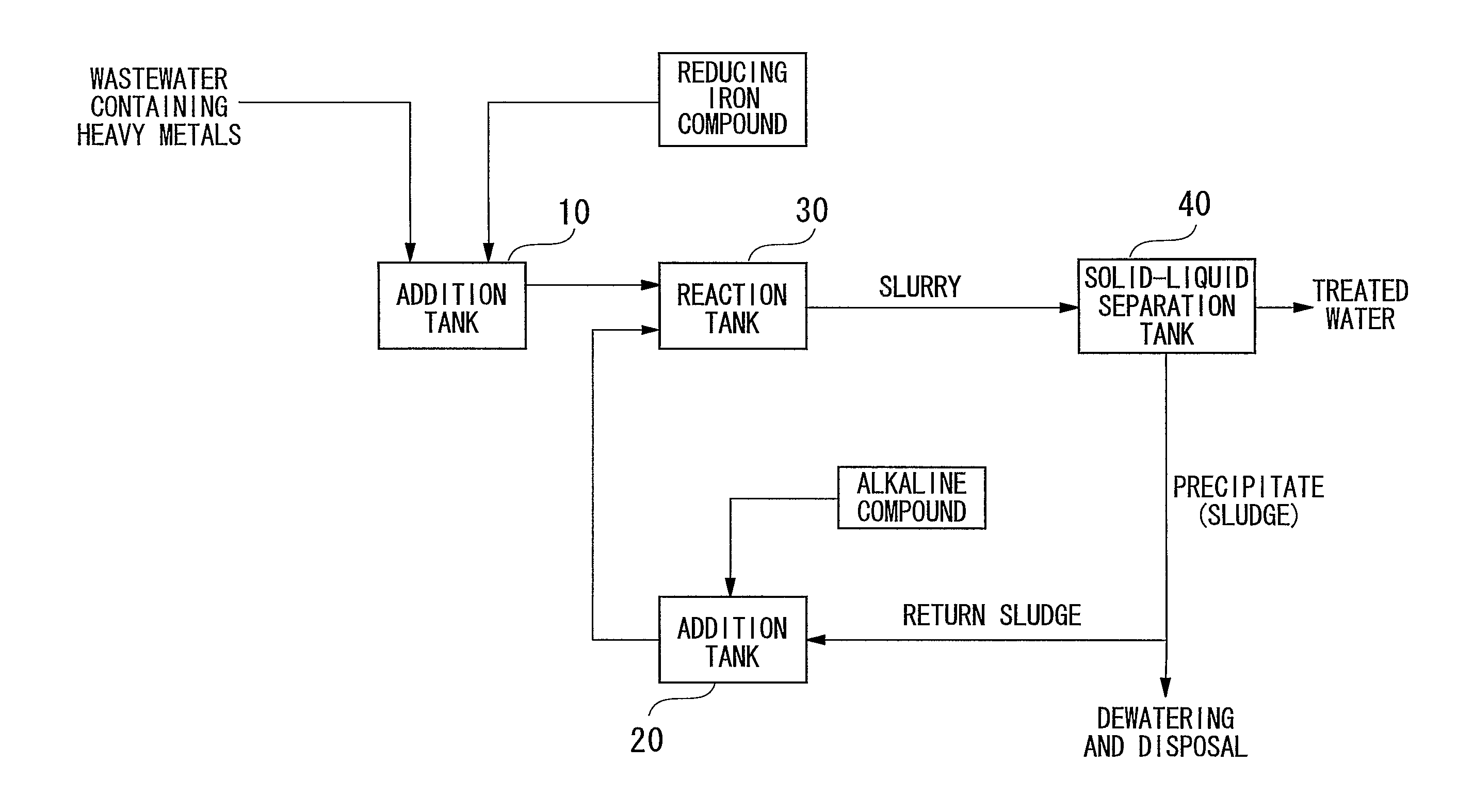
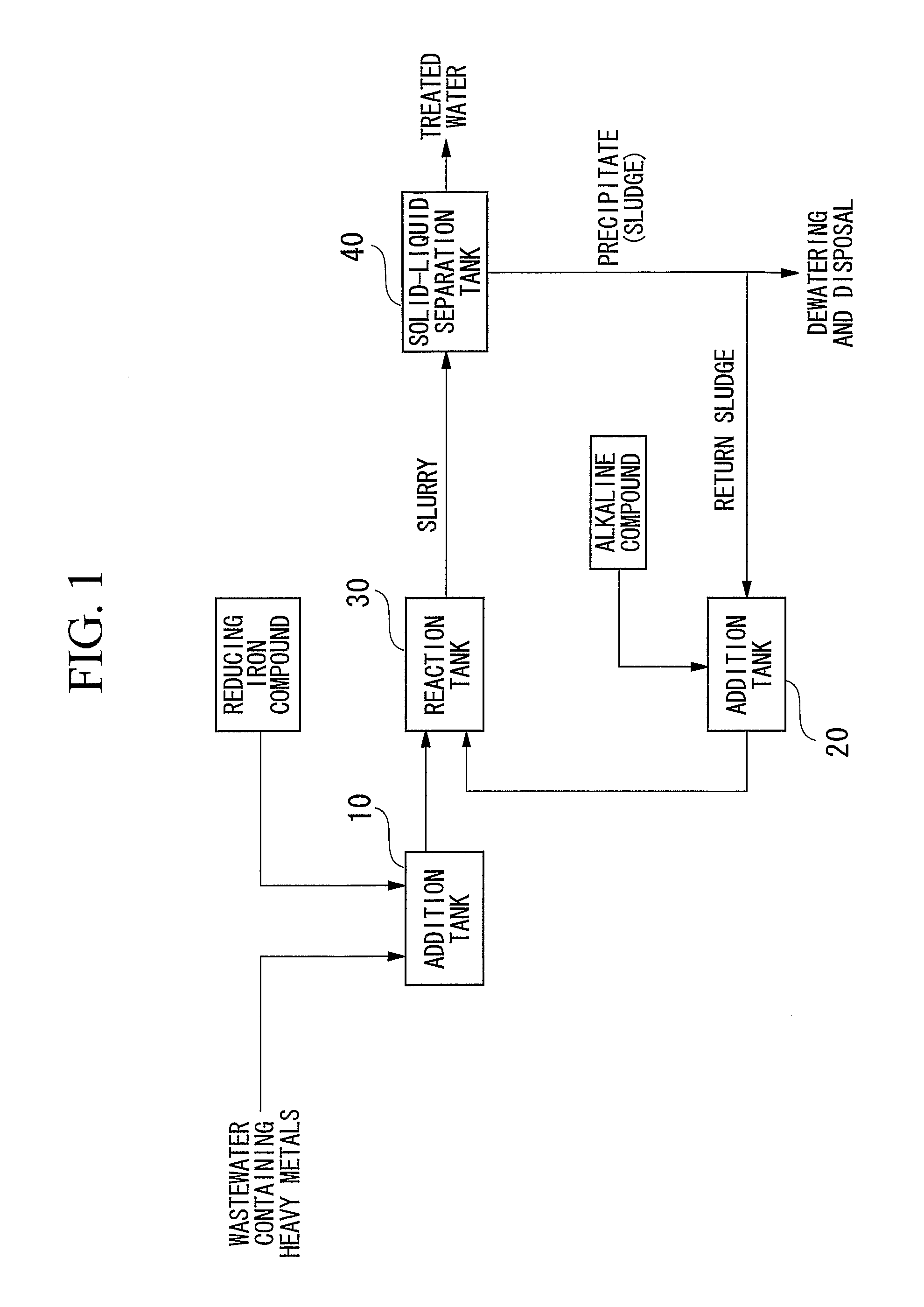
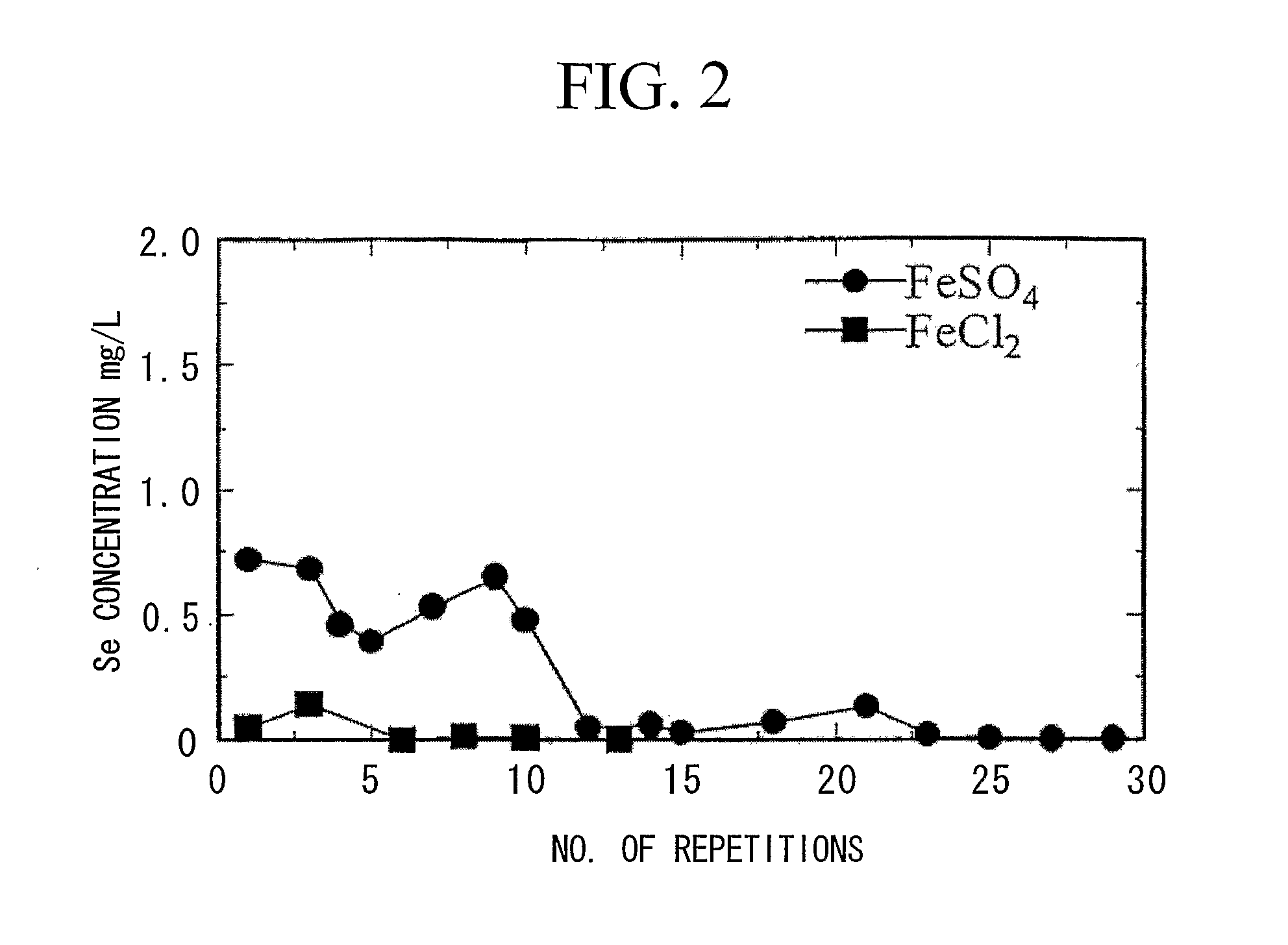
A reducing water purification material having a reducing iron-based precipitate selected from green rust, iron ferrite, reducing iron hydroxide, and a mixture thereof. A wastewater treatment process having steps of adding a reducing iron compound to wastewater, leading the wastewater to which the reducing iron compound is added to a reaction tank and forming a precipitate, separating the formed precipitate by a solid-liquid separation to obtain a sludge, and alkalinizing all or a portion of the separated sludge to form an alkaline sludge followed by returning to the reaction tank, wherein in the precipitation step, the wastewater to which the reducing iron compound is added and the alkaline sludge are mixed and are allowed to react in a non-oxidizing atmosphere under alkaline condition to form a reducing iron compound precipitate as the precipitate, thereby incorporating contaminants in the precipitate to remove the contaminants from the wastewater.
Owner:MITSUBISHI MATERIALS CORP
System for treating heavy metal wastewater
InactiveCN102503030AGive full play to the adsorption effectEasy to handleWater contaminantsMultistage water/sewage treatmentAbsorption columnSorbent
The invention discloses a system for treating heavy metal wastewater. The system comprises an aeration pond, a neutralization pond, a flocculation pond, a thickening pond, a mechanical filtering tank, an absorption column, and functional wetland which are connected sequentially, and also comprises a ferric salt feeding system and a sludge backflow system. The concentration of Fe<3+> in wastewateris improved by aerating and feeding ferric salt, and the concentration of lead, arsenic and cadmium in a solution is reduced by using a Fe<3+> co-precipitation effect, and an adsorption effect of active iron hydroxide; by backflow of sludge, the feeding amount of lime is reduced by 5 to 10 percent, and the water treatment capacity is improved by 1 to 3 times; a high-flux large-aperture resin loaded with ferric hydroxide is used as adsorbent which has a strong effect of adsorbing the lead, arsenic and cadmium in wastewater, and effluent meets the requirement of V-grade water body of 'environmental quality standard of surface water' (GB3838-2002); and the functional wetland fully exerts the adsorption effect of filler on heavy meal, and an enrichment effect of plant, and the effluent reaches the requirement of III-grade water body.
Owner:CHINA UNIV OF MINING & TECH (BEIJING)
Method for preparing carbon coated nano metal material using biological material-starch as substrate
The present invention belongs to the field of the crossed nano carbon material and biological material technology, and relates to the temperature control carbonizing process for preparing carbon coated nano material under hydrogen atmosphere with starch as precursor material. Starch as carbon source precursor and ferric hydroxide prepared with ferrite nitrate are mixed homogeneously and the mixture is dried and ground to obtain material pre-mixture. The material pre-mixture is carbonized under reduction amtosphere and controlled temperature to prepare carbon coated nano material material. The present invention has low cost, simple process, controllable condition and other advantags, and the product may be large scale produced and widely used in high density magnetic record material, magnetic fluid nuclear magnetic resonant imaging and other fields.
Owner:CHANGZHOU INST OF DALIAN UNIV OF TECH
Method for preparing magnetic compound material of ferric oxide cladded carbon nanotube
InactiveCN1670251AImprove performanceMeet the needs of developmentMetallic material coating processesTransformerCarbon nanotube
The invention discloses a method for preparing magnetic composite material of a carbon nanometer tube covered with di-iron trioxide. The invention makes the carbon nanometer tube reflux in the nitric acid, to introduce several function groups such as carboxyl group, hydroxyl group and carbonyl group in the tube wall, which can adsorb iron ions in the solution. Then the iron ions adsorbed in the tube wall react with the hydroxide ion in the ammonial solution, which coated the hydroxide ion on the tube, and the tube can be coated with di-iron trioxide by calcining. The invention provides a simple way to get composite material, good performance soft magnetic material, which is facilitating to industrial production, and may be used in the areas of high-density magnetic recording material, wave-absorbing material, ink for electrophotographic copying, broad-cellulae transformer, noise filter, voltage transformer for communicating and so an.
Owner:DONGHUA UNIV
Production method of ammonium ferric citrate
ActiveCN101704738AAvoid generatingIncrease productivityCarboxylic acid salt preparationImpurityAmmonium ferric citrate
The invention discloses a production method of ammonium ferric citrate. The ammonium ferric citrate is prepared by water, citric acid and iron powder according to the following steps: 1. placing water and citric acid into a stirring device to be heated to 60 DEG C; 2. opening the stirring device, adding the iron powder, slowly heating to 80 DEG C, keeping the temperature, stirring and reacting to generate ferrous citrate; 3. cooling the ferrous citrate obtain in step 2 to 40 DEG C, adding hydrogen peroxide for oxidizing until no ferrousion exists; 4. introducing ammonia to neutralize the ferrate compound obtained in step 3 to the pH value more than or equal to 7, filtering, removing impurities and concentrating to be in a pasty shape; and 5. placing the concentrated matter obtained in step 2 into an oven for drying at the temperature of 80 DEG C. The invention has the advantages of adopting the iron powder to replace ferrous sulphate, using the hydrogen peroxide as oxidant and adopting scientific proportioning and production process, thus avoiding chloridion and sulfate ion and other impurities from being carried in, preventing from generating ferric hydroxide intermediate products which are difficult to dehydrate and wash, greatly simplifying production processes, reducing production cost and improving production efficiency of the ammonium ferric citrate.
Owner:郑州瑞普生物工程有限公司
Preparation method for amorphous FeOOH water-purifying agent
InactiveCN105800762AHigh arsenic removal efficiencyEfficient removalWater/sewage treatmentAluminum IonHydrolysis
The invention provides a preparation method for an amorphous FeOOH water-purifying agent. The preparation method comprises the following steps: preparing a ferrous sulfate solution from industrial ferrous sulfate heptahydrate; adding a proper amount of sulfuric acid into the ferrous sulfate solution so as to provide an acidic environment; adding hydrogen peroxide into the solution and allowing ferrous sulfate to be oxidized into ferric sulfate under the condition of hydrolysis promotion by sulfuric acid; subjecting an industrial alkali source and the ferric sulfate solution to a precipitation reaction so as to produce iron hydroxide colloid; and dehydrating the iron hydroxide colloid under proper conditions so as to prepare the amorphous FeOOH water-purifying agent. The amorphous FeOOH water-purifying agent can effectively arsenic in water, has arsenic removal efficiency of 98% or above, does not contain aluminum ions or pose secondary pollution to a water body; after arsenic removal with the water-purifying agent, scorodite stably existing in the nature is produced, so pollution is not posed to the environment; moreover, raw materials used in the invention are of an industrial grade, and the preparation method is low in production cost and simple to operate.
Owner:BEIJING SJ ENVIRONMENTAL PROTECTION & NEW MATERIAL CO LTD
Process for purifying lithium chloride
InactiveCN1483673AImprove adsorption capacitySolve the industrial problem of removing impurity sodiumLithium halidesLithium chlorideLithium hydroxide
A process for purifying lithium chloride (LiCl) from industry grade LiCl and hydrochloric acid includes mixing the above raw materials, adding barium chloride and lithium hydroxide to PH=9-13, adding lithium oxalate or oxalic acid under normal temperature and stir to get the mixture of precipitation of barium sulfate, calcium oxalate, magnesium hydroxide and ironic hydroxide, passing the mixture through a membranous filter to get rid of the ions of sulfate, calcium, magnesium and iron, adding a reagent LF with a composition of Li1+xAxB2-x(PO4)3 under normal temperature, ordinary pressure, and PH9-13, purifying 6-96 hours with stir, passing it through a membranous filter again, concentrating and drying. The pureness of lithium chloride obtained is higher than 99.9%, and Na<30ppm, K<10ppm, Ca<20ppm, Mg<10ppm, Fe<5ppm.
Owner:NORTHEASTERN UNIV
Method for producing calcium chloride dihydrate
InactiveCN101683997ANo pollution in the processHigh recovery rateCalcium/strontium/barium chloridesEmulsionCalcium Chloride Hexahydrate
The invention relates to a method for producing calcium chloride dihydrate, which is characterized by comprising the following steps: putting hydrochloric acid (31%) and limestone powder into a reaction vat according to the mixture ratio of 2.2:1, and stirring for reaction to generate an acid calcium chloride solution; transferring the obtained solution into a clarification tank, adding lime emulsion, regulating the pH value of the solution to 8.9-9 to precipitate out iron hydroxide and magnesium hydroxide; and transferring filtrate into an evaporation dish after clarifying and filtering, heating to the temperature of 172-174 DEG C, evaporating, drying and dewatering at the temperature of 200-240 DEG C after crystallizing and separating, to obtain the calcium chloride dihydrate. The invention has the following advantages of no pollution, high recovery rate and product cost reduction.
Owner:TIANJIN LIHONG CHEM TECHN
Method for preparing 1,3,3,3-tetrafluoropropene by gas phase fluorination
ActiveCN101913985AHigh reaction yieldPhysical/chemical process catalystsPreparation by halogen replacementHydrogen fluorideGas phase
The invention discloses a preparation method of 1,3,3,3-tetrafluoropropene. The method takes 1-Cl-3,3,3-trifluoropropene as a raw material and comprises the following steps of: performing the reaction of hydrogen fluoride (HF) and the 1-Cl-3,3,3-trifluoropropene under the catalytic action of a fluorinated catalyst, wherein the reaction conditions are as follow: the mol ratio of the HF and the 1-Cl-3,3,3-trifluoropropene is 5-15:1, the contact time is 2 to 20 seconds, the reaction temperature is 300 to 450 DEG C, precursors of the fluorinated catalyst comprise 60 percent by mass of calcium fluoride, 30 percent by mass of iron hydroxide and 10 percent by mass of calcium carbonate, and the fluorinated catalyst can be prepared by evenly mixing the precursors of the fluorinated catalyst, pressing and moulding, roasting at 450 DEG C and carrying out the fluorination by using hydrogen fluoride gas at 400 DEG C. The invention is mainly used for preparing the 1,3,3,3-tetrafluoropropene.
Owner:XIAN MODERN CHEM RES INST
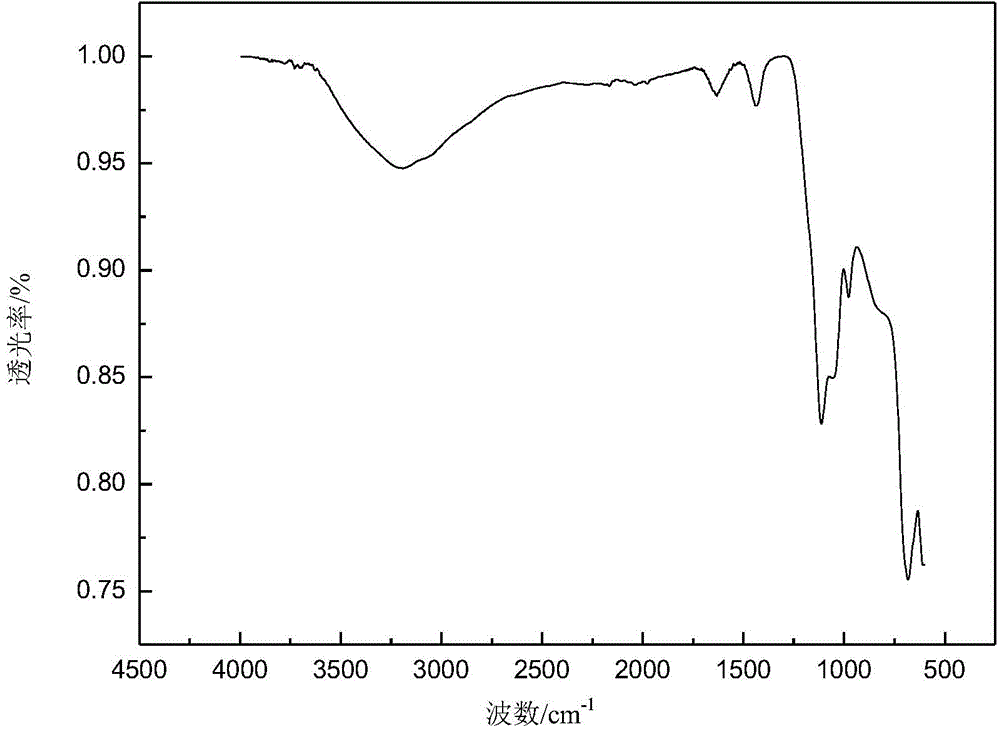
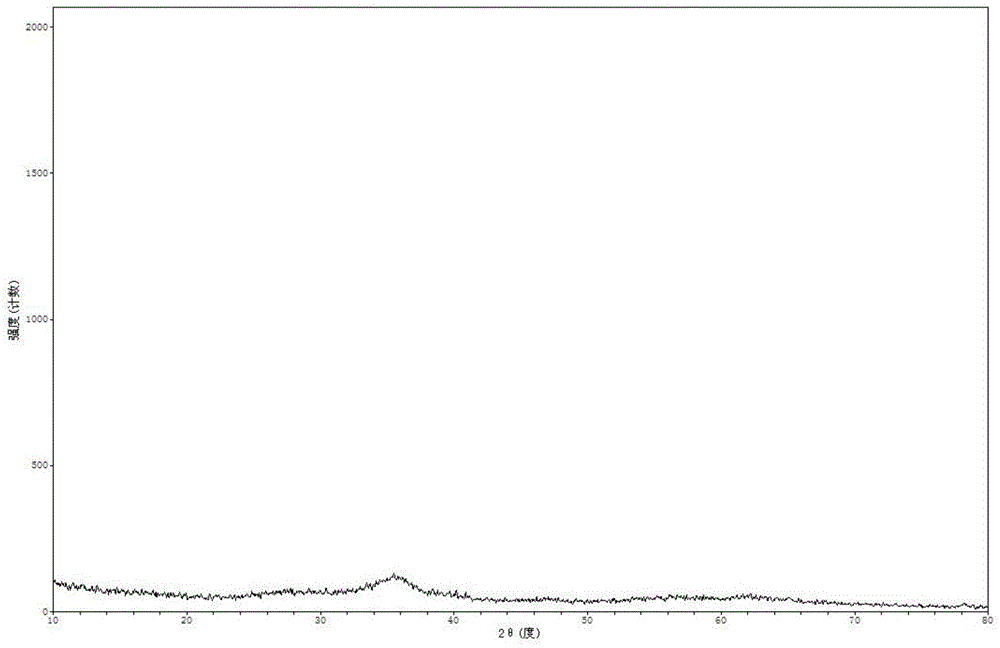
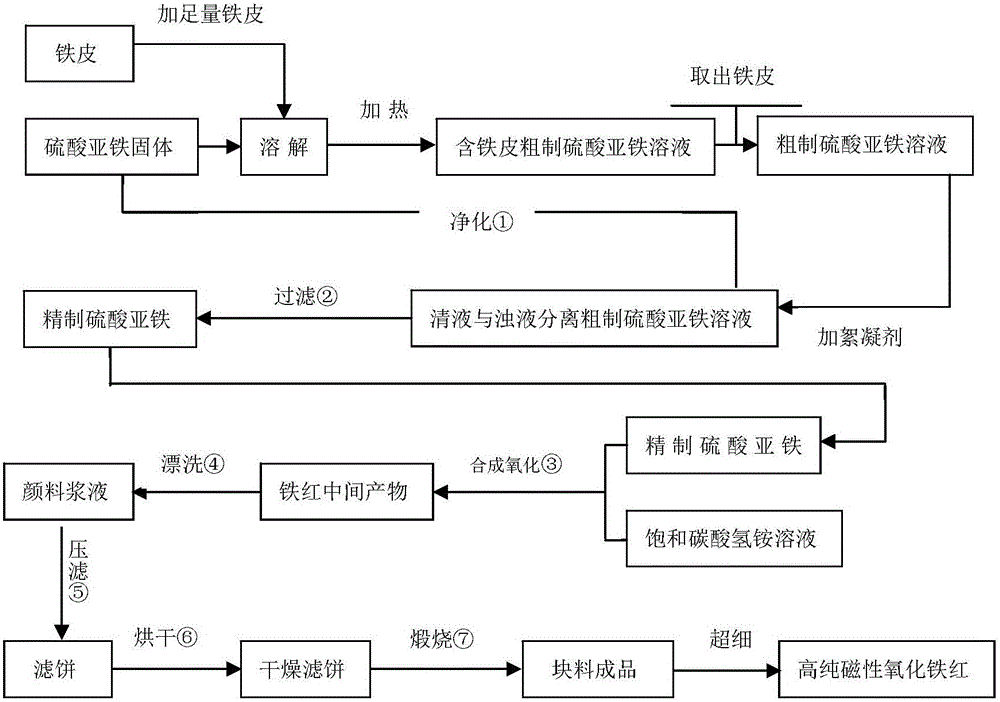
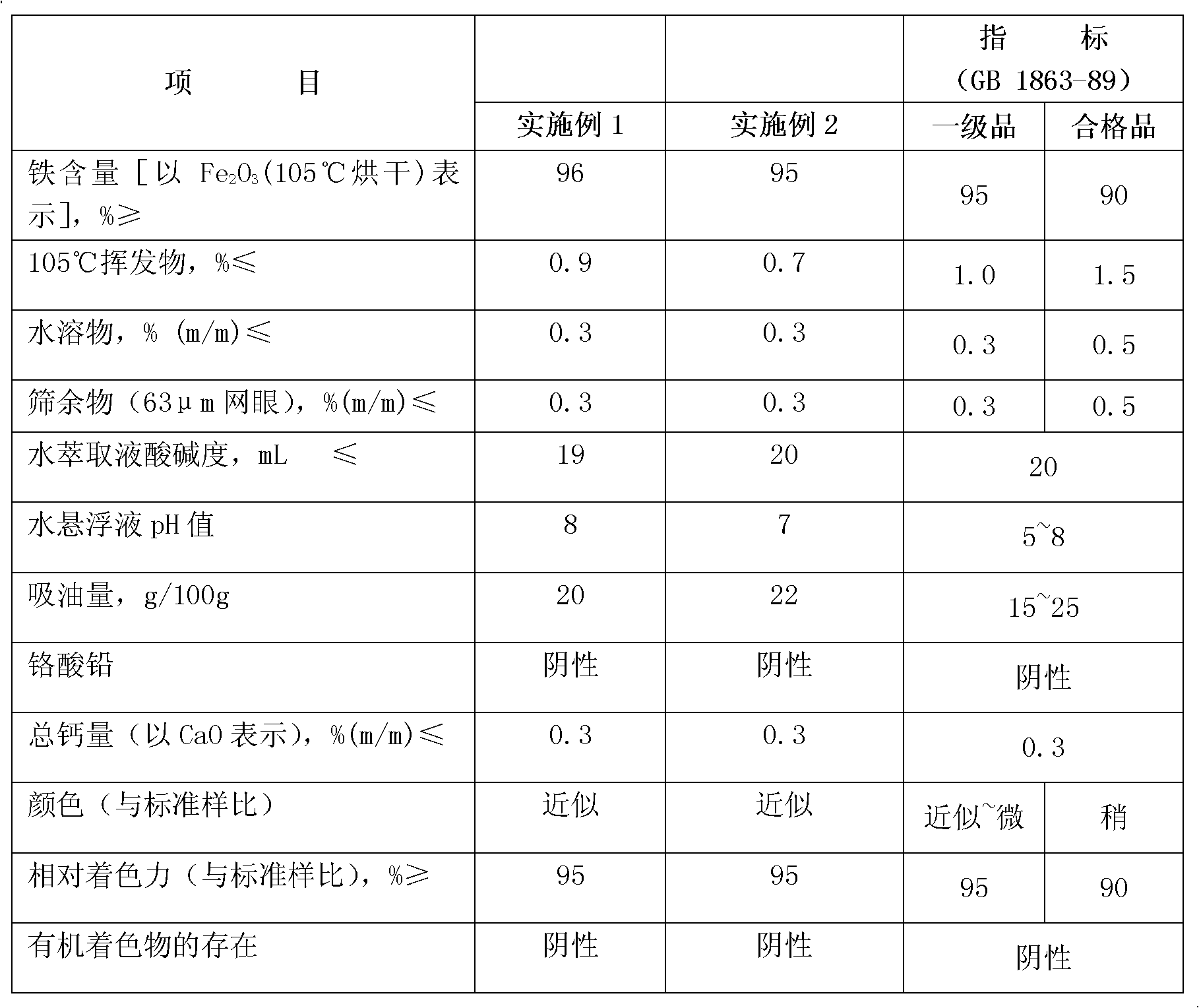
Method for preparing high-purity gamma-Fe2O3 iron oxide red pigment
InactiveCN105110382AEasy to filter washNo crushing requiredFerric oxidesSulfateFerric hydroxide oxide
The invention relates to a method for preparing high-purity gamma-Fe2O3 iron oxide red pigment. The method includes the following steps: firstly, dissolving the ferrous sulfate solid in water, controlling the obtained solution to be at a certain temperature, adding a certain amount of a flocculating agent, and keeping at a certain temperature; after a certain time, filtering to obtain a refined ferrous sulfate solution; adding saturated ammonium bicarbonate solution into the refined ferrous sulfate solution for synthesizing iron carbonate and iron hydroxide precipitate with high magnetic properties under the conditions of a certain temperature and a pH value. According to the invention, the method is low in cost and simple and convenient to operate; the obtained product is high in stability and suitable for industrial production.
Owner:ZHEJIANG HUAYUAN PIGMENT CO LTD
Method for preparing ferric oxide red pigment by using nitryl chloride tail gas
ActiveCN102180521AReduce pollutionAchieve sustainable developmentFerric oxidesNitryl chlorideNitro reduction
The invention discloses a method for preparing ferric oxide red pigment by using nitryl chloride tail gas. The nitryl chloride tail gas is waste gas from the production process of high temperature chlorination of chloronitrobenzene. The method comprises the following steps of: adding chemical iron mud into a water absorption cell of the nitryl chloride tail gas; introducing steam to heat the water to 90 to 100 DEG C, stirring to dissolve the chemical iron mud; tracing and measuring the Fe3<+> content and the added quantity of the chemical iron mud in the solution; after the Fe3<+> content in the solution reaches 110 to 130g / L, filtering the solution, and adding ammonia water into the filtrate, adjusting the pH value to 6 to 9 and allowing trivalent iron to form iron hydroxide; precipitating; performing centrifugal separation on the precipitate; heating the solid to 105 to 115 DEG C; and decomposing the solid to obtain the ferric oxide red pigment, wherein the chemical iron mud is obtained after the nitro reduction reaction of iron powder. In the method, the harmful nitryl chloride tail gas and the solid waste iron mud obtained after nitro-reduction are used as raw materials to prepare the ferric oxide red pigment, so waste materials are changed into valuable materials, and the aims of sustainable development, energy conservation, consumption reduction and environmental pollution reduction are fulfilled.
Owner:海宁市黄湾镇资产经营有限公司
High-efficient aiding burn environmental protection coal saving agent
The invention is a high-performance combustion-supporting environmental-protection coal-saving agent, including the following components in weight percent: MgCl2 5-12%, sodium phosphate 6-15%, potassium permanganate 0.8-1.2%, iron hydroxide 0.1-0.3%, and magnesium hydroxide0.1%, and its formula is reasonable and its synthetic cost is low. As used in a boiler, it can make bunker coal fully burned, increasing combustion heat by 15-25%, reducing the quantity of CO, CO2 and smoke dust discharged into the atmosphere and reducing the pollution of harmful substances to the atmosphere environment.
Owner:高保安
Method for preparing alkyl aromatic ferric sulfonate
The invention relates to a method for preparing alkyl aromatic ferric sulfonate, which comprises the following steps of: carrying out back flowing reaction of ferric hydroxide and alkyl aromatic sulfonic acid in water solution with the mol ratio of ferric hydroxide to alkyl aromatic sulfonic acid being 1 / 2 to 1 / 4; carrying out spray drying at the temperature between 120 DEG C and 300 DEG C to obtain crude products of the alkyl aromatic ferric sulfonate; washing the generated crude products with diethyl ether; removing the excessive alkyl aromatic sulfonic acid; and then, removing the diethyl ether and the water through vacuum distillation to obtain finished products. The method avoids the introduction of chloridion in the preparation process, is quite suitable for mass production, and is more applicable to solid-state electrolytic capacitor industry in the electronic industry.
Owner:胡思棉
Method of producing magnesium hydroxide and light magnesium oxide using magnesite as raw material
InactiveCN1868952AImprove resource utilizationNo pollutionMagnesium hydroxideLime productionResource utilizationSlurry
A process for preparing magnesium hydroxide and light magnesium oxide from magnesite includes such steps as calcining the magnesite in carbonizing kiln to obtain magnesium oxide, preparing magnesium oxide slurry, carbonizing to become magnesium carbonate, acidolyzing to become soluble magnesium hydroxide, adding oxidant to the mixed acid solution for oxidizing the ferrous elements to become iron hydroxide, filtering for removing it, adding soluble magnesium hydroxide and ammonium salt solution, thermodecomposing to generate magnesium hydroxide, press filtering, and light burning or baking.
Owner:任建武
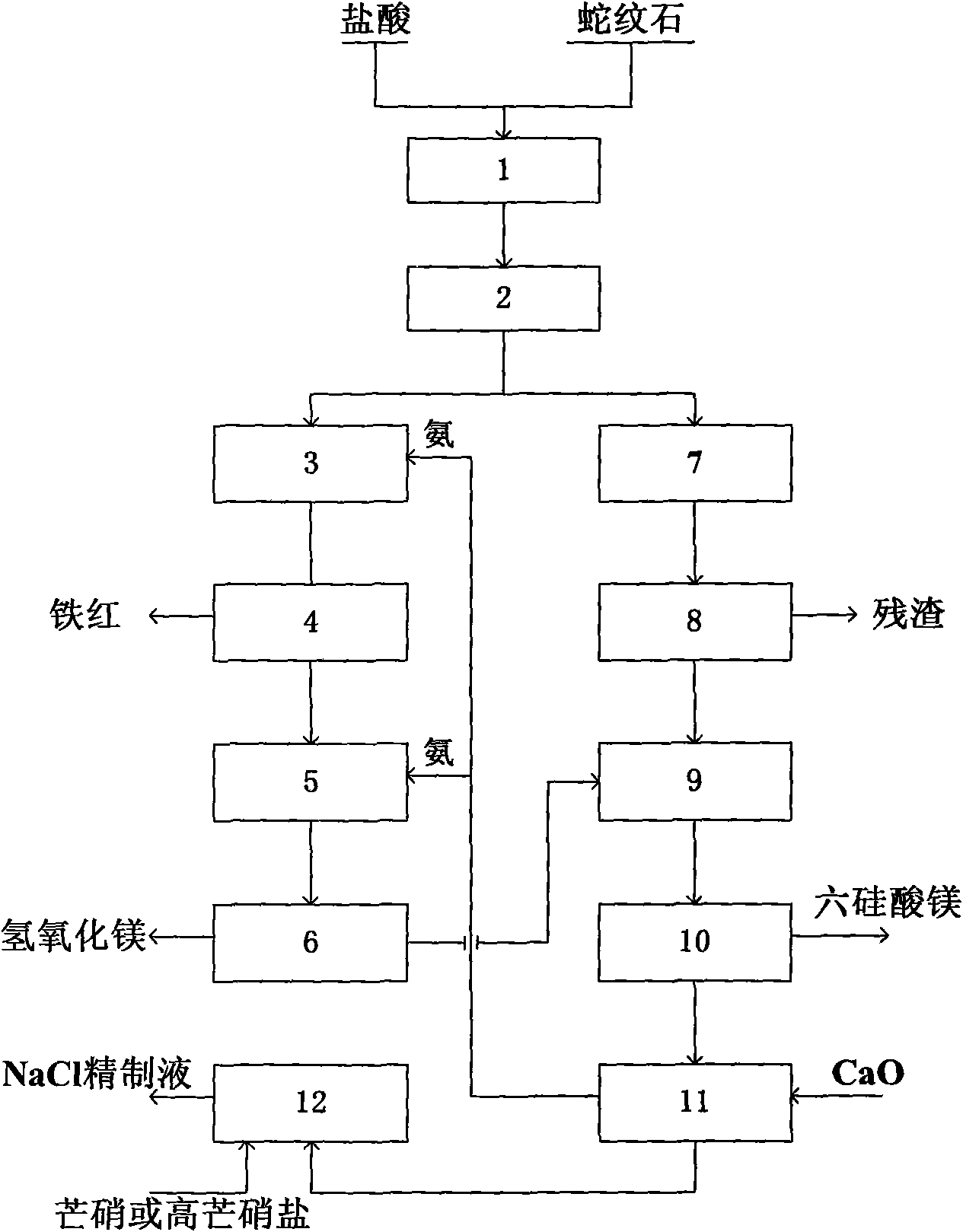
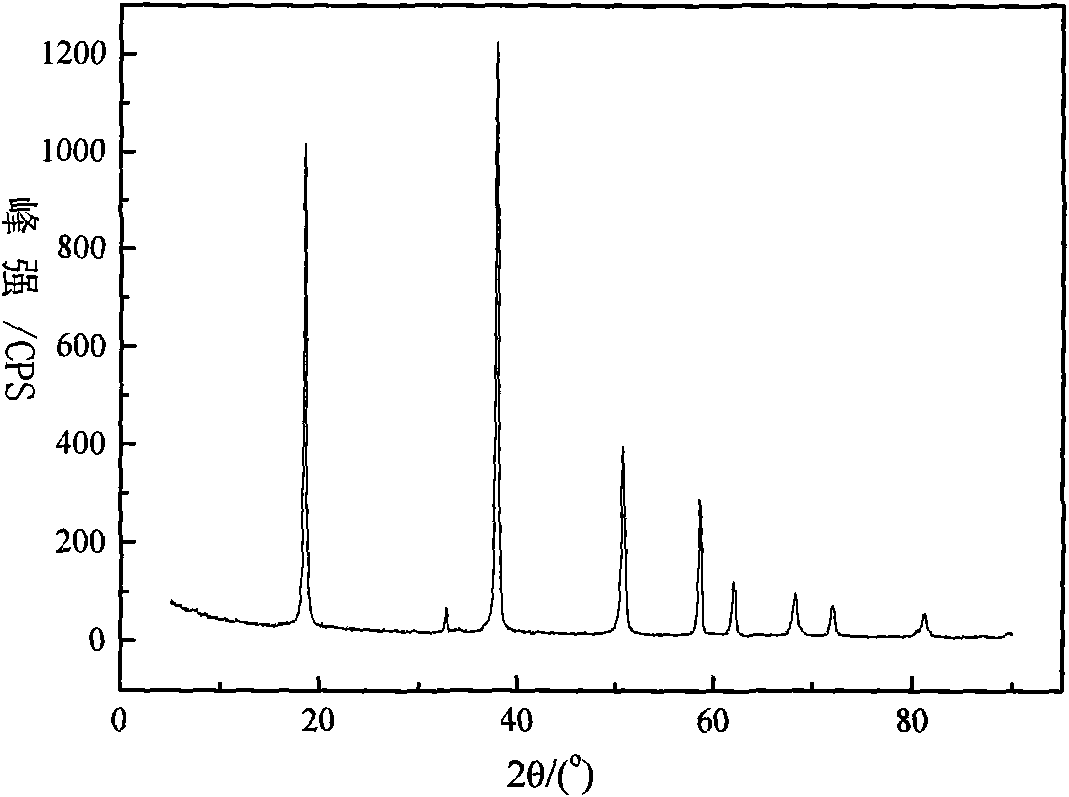
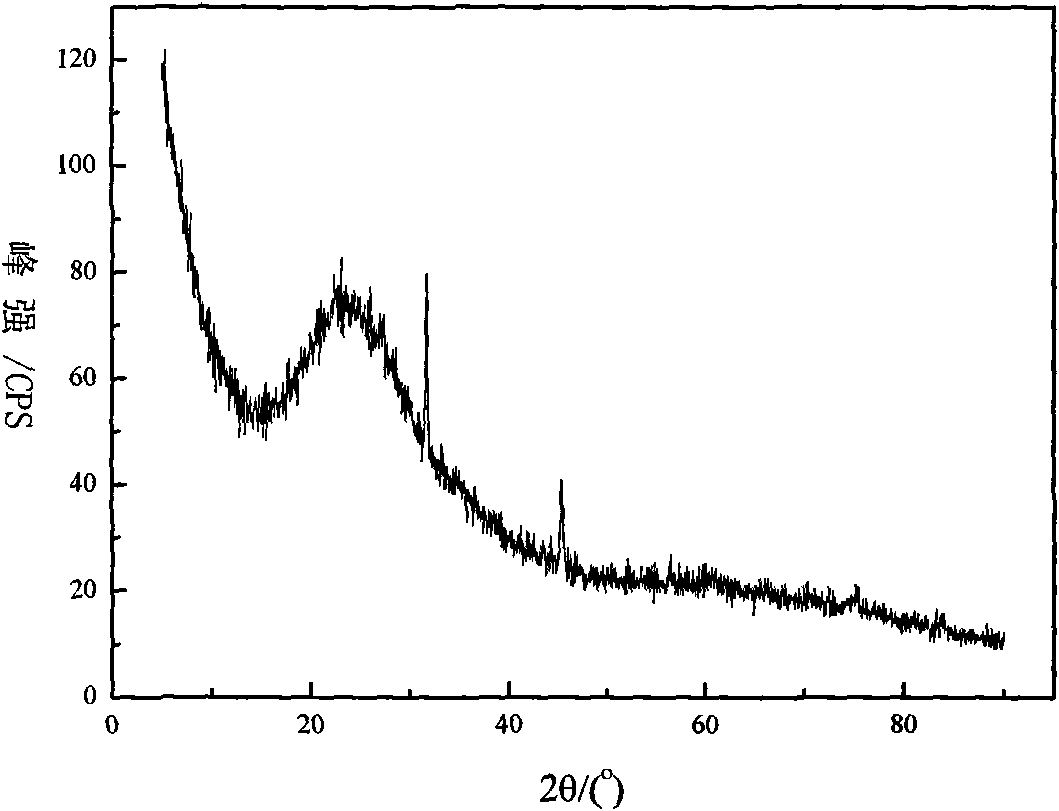
Method for preparing highly pure magnesium hydroxide and magnesium hexa-silicate by using olivine tailing mine
InactiveCN101607721AGuaranteed cycleReduce manufacturing costMagnesium silicatesSolid waste disposalSlurryOlivine
The invention discloses a method for preparing highly pure magnesium hydroxide and magnesium hexa-silicate by using olivine tailing mine, which comprises the following steps: step one. olivine tailing mine and muriatic acid are blended, leached and reacts; step two. pickle liquor leached by acid is added into an enamel reactor and oxidized by adding oxidant hydrogen peroxide; step three. the component of a solid product after reaction is a ferric oxide which is washed and dried after solid-liquid separation to be iron oxide red; step four. filtrate after solid-liquid separation is continously added with ammonia water; step five. sizing agent containing magnesium hydroxide stays in reactant, aged, washed, dried and ground to obtain highly pure magnesium hydroxide; step six. solid leached by muriatic acid reacts with caustic soda to produce serous fluid containing sodium silicate; and step seven. reaction product which reacts with caustic soda solution is filtered and washed to obtain magnesium hexa-silicate. The method causes magnesium oxide to be fully recycled. The method ensures complete circulation of ammonia, increases yield of magnesium oxide in mineral, reduces the cost of magnesium hydroxide, and simultaneously ensures the purity of the magnesium hydroxide which reaches more than 99%.
Owner:宜昌弘林华镁矿业投资有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com