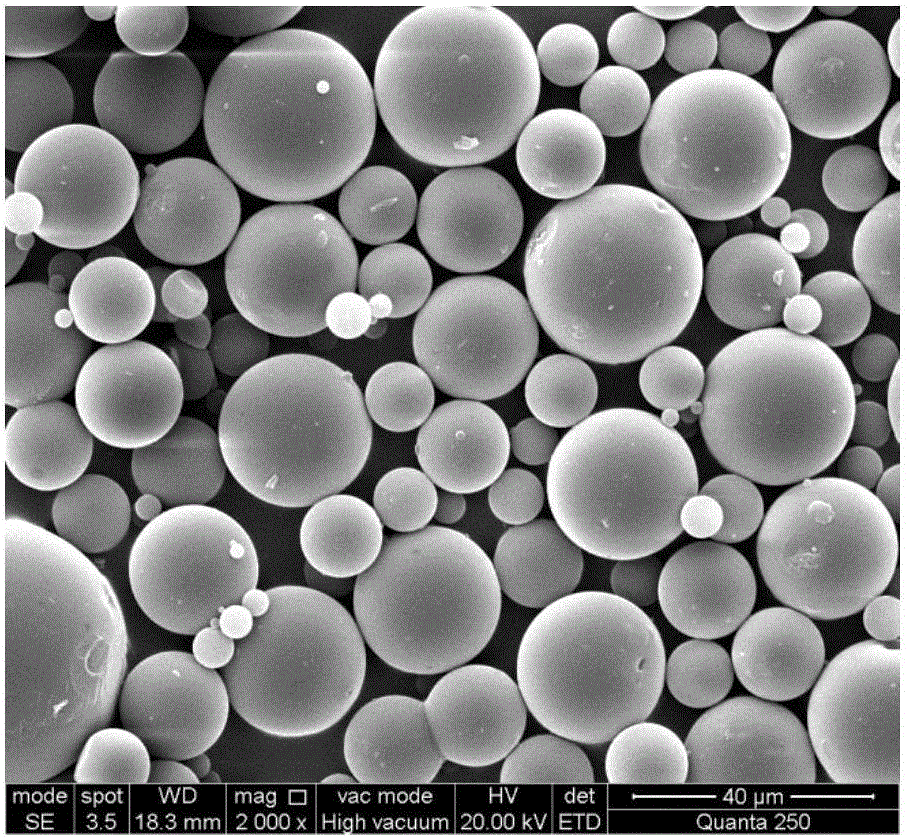
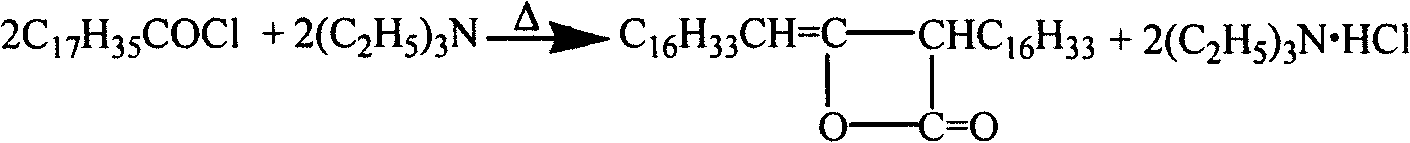Patents
Literature
234 results about "Calcium Chloride Hexahydrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Calcium Chloride Hexahydrate is an excellent water soluble crystalline Calcium source for uses compatible with chlorides. Chloride compounds can conduct electricity when fused or dissolved in water. Chloride materials can be decomposed by electrolysis to chlorine gas and the metal.
Composite amorphous phase-change heat storage material and preparation method thereof
InactiveCN103525373AReduce leakageLeakage is not easy to happenHeat-exchange elementsActive agentHeat conducting
The invention relates to a composite amorphous phase-change heat storage material and a preparation method thereof, and belongs to the technical field of composite materials. The composite material is prepared from a porous material used as a support material and an inorganic hydrous salt used as a phase-change material in manners of absorbing the inorganic hydrous salt into a porous structure of the support material by virtue of the capillary adsorption action of the porous material, and self-assembly of a surfactant. The method comprises the following steps: firstly, emulsifying and dispersing the molten inorganic hydrous salt phase-change material such as calcium chloride hexahydrate by the emulsifying dispersion effect of the surfactant under an ultrasonic condition, so as to form a stable emulsion; adding expanded graphite to the emulsion to evenly disperse; putting the mixture into a vacuum oven, vacuumizing and keeping in a constant state for 30 minutes at room temperature, so as to prepare a novel composite amorphous phase-change heat storage material which is high in phase-change latent heat, good in heat-conducting property, and low in cost.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Calcium chloride hexahydrate phase change energy storage material composition
InactiveCN102134473AHigh latent heat of phase changeGood temperature regulationHeat-exchange elementsCalcium Chloride HexahydrateEnergy storage
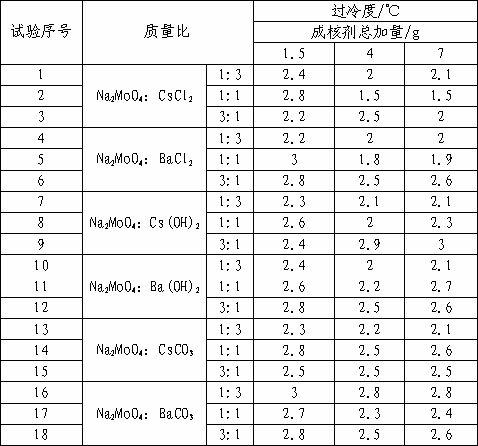
The invention discloses a calcium chloride hexahydrate phase change energy storage material composition, which comprises calcium chloride hexahydrate, a nucleating agent, a thickener, other functional additives and the like. The phase change temperature of the composition is 23 to 25 DEG C, the degree of supercooling is less than 3 DEG C, and the latent heat of phase change is more than 180kJ / kg. The composition has high phase change stability, passed 3,000 times of tests of cold and hot circulation and can keep the uniform distribution of the nucleating agent in a short time period.
Owner:益田润石(北京)化工有限公司
Compound chloride-based environment-friendly snowmelt agent
InactiveCN101691481AAvoid Decomposition DefectsEasy to operateOther chemical processesSodium metasilicateDecomposition
The invention relates to a compound chloride-based environment-friendly snowmelt agent, which comprises a main component of a chloride mixture of calcium chloride dihydrate and magnesium chloride hexahydrate, contains various additives of sodium citrate, disodium hydrogen phosphate, sodium metasilicate, urea and the like and can contain abscisic acid or indoleacetic acid or a mixture thereof, sodium tartrate, humic acid or a salt thereof. The snowmelt agent in the invention can reduce the corrosivity to a metal object and a concrete structure effectively, improves the anti-salinization capabilities of a seed, a plant and soil, can be used when the temperature is as low as -25 DEG C, and solves the problems of bad snow melting effect caused by an autologous freezing point characteristic when a sodium chloride-based snowmelt agent is used and high cost caused by using an anhydrous calcium chloride-based snowmelt agent under the condition. The invention also provides a use method of the snowmelt agent, which places the main component and each additive separately without preparing a finished product of the snowmelt agent in advance and compounds the snowmelt agent on the spot before using. The method can avoid the problems of wetting, hardening and the like of the finished product of the snowmelt agent in the processes of storing and transporting, can avoid the defects of high-temperature energy consumption in the process of preparing compound granules (such as coating and granulating) and the capability of causing decomposition of the additives, has simple operations and a low cost, and is particularly suitable for on-site mechanical shed work.
Owner:CHINA ACAD OF TRANSPORTATION SCI +1
Anhydrous aluminum chloride production method
InactiveCN104773746ASolve the problem that the purity is also very low and it is difficult to meet the industrial requirementsAluminium chloridesAluminium chlorideCalcium Chloride Hexahydrate
The invention relates to an anhydrous aluminum chloride production method. In the prior art, the aluminum chloride product produced by using the hydrochloric acid dissolution method has disadvantages of fine particle, large specific surface area and high impurity content, does not meet metallurgical grade alumina requirements, and is difficultly be subjected to direct use, and the prepared aluminum chloride purity is low and difficultly meets the industrial requirements even the aluminum chloride product is utilized. With the production method of the present invention, the problems in the prior art are solved. The production method comprises: immersing an aluminum production raw material into hydrochloric acid to obtain an aluminum chloride solution; directly carrying out concentration crystallization on the prepared aluminum chloride solution to obtain an aluminum chloride hexahydrate crystal; calcining the aluminum chloride hexahydrate crystal to obtain primary alumina; and mixing the primary alumina and carbon, adding to a chlorination furnace, introducing chlorine gas, heating, carrying out a reaction of the alumina and the chlorine gas to generate gaseous aluminum chloride, and carrying out refining impurity removing to obtain the pure anhydrous aluminum chloride. The production method of the present invention is used for industrial production of the anhydrous aluminum chloride.
Owner:GUIYANG AL-MG DESIGN & RES INST
Inorganic hydrated salt phase change energy storage microcapsule and preparation method thereof
InactiveCN106244117AIncrease coverageSimple processHeat-exchange elementsCalcium Chloride HexahydratePotassium fluoride
Belonging to preparation methods of energy storage microcapsule materials, the invention provides an inorganic hydrated salt phase change energy storage microcapsule and a preparation method thereof. The energy storage microcapsule includes an inorganic hydrated salt serving as the core material and an inorganic material serving as the wall material. The core material is one or more of potassium fluoride dehydrate, sodium acetate trihydrate, sodium thiosulfate pentahydrate, calcium chloride hexahydrate, magnesium sulfate heptahydrate, barium hydroxide octahydrate, sodium sulfate decahydrate, sodium sulfate decahydrate, disodium hydrogen phosphate dodecahydrate, ammonium aluminium sulfate dodecahydrate, aluminum potassium sulfate dodecahydrate, and aluminum sulphate ocatadecahydrate. The wall material is one or more of silicon dioxide, calcium carbonate, alumina and titanium dioxide. The core material accounts for 30%-80% of the mass of the microcapsule composite material, and the wall material accounts for 20%-70% of the mass of the microcapsule energy storage material. The prepared phase change energy storage microcapsule material has a phase transition temperature of 25-100DEG C and a diameter of 0.1-50 micrometers. The phase change energy storage microcapsule has the advantages of high encapsulation rate, good sealing performance, large phase change potential heat value, and simple preparation method, and has great industrial application prospect.
Owner:CHINA UNIV OF MINING & TECH
Inorganic hydrous salt phase change microcapsule energy-storage material and preparing method
InactiveCN106221674AAchieve solidificationImprove heat transfer efficiencyHeat-exchange elementsCalcium Chloride HexahydrateSodium Thiosulfate Pentahydrate
The invention relates to a preparing method of an inorganic hydrous salt phase change microcapsule energy-storage material and belongs to preparing methods of energy-storage materials. The energy-storage material comprises a core material and a wall material, wherein the core material is prepared from one or more of calcium chloride hexahydrate, sodium sulfate decahydrate, sodium thiosulfate pentahydrate, disodium hydrogen phosphate dodecahydrate, sodium acetate trihydrate and sodium carbonate decahydrate inorganic hydrous salt, and the wall material is prepared from one or more of polystyrene, polymethyl methacrylate, poly(ethyl acrylate), polyurethane, cellulose acetate butyrate (CAB) and a diphenylmethane diisocyanate polymer, the core material accounts for 30-80% of the microcapsule energy-storage material by mass, and the wall material is prepared from the polymer and accounts for 20-70% of the microcapsule energy-storage material by mass. The phase change point of the obtained phase change microcapsule energy-storage material ranges from 20 DEG C to 90 DEG C, and the particle size ranges from 1 micrometer to 100 micrometers. The phase change microcapsule energy-storage material prepared with the method is high in encapsulation rate, good in sealing performance, large in phase change latent heat value, simple in preparing method and large in industrial application prospect.
Owner:CHINA UNIV OF MINING & TECH
Drying agent
ActiveCN103566910ALow costWon't seepOther chemical processesDispersed particle separationLiquid waterCalcium Chloride Hexahydrate
The invention belongs to the field of gas drying, and provides a drying agent. The drying agent comprises 1-5 parts by weight of anhydrous calcium chloride, 0-8 parts by weight of calcium chloride dihydrate, 1-5 parts by weight of polyacrylamide and 0-1 part by weight of vegetable starch by weight. The drying agent provided by the invention adopts an organic macromolecule component, and is low in raw material cost; higher hygroscopicity can be obtained and can be up to over 400% through the structural characteristics of macromolecules; the drying agent is long in effective time; no liquid water oozes.
Owner:KUNSHAN WEISHENG DRIER
Controlled release of surfactants for enhanced oil recovery
ActiveUS8946132B2Reduce surface tensionEnhanced overall recoveryFlushingDrilling compositionCalcium Chloride HexahydrateMetal salts
A controlled release composition comprising an aqueous sulfonate solution; an anionic surfactant; and a salt selected from aluminum nitrate nanohydrate, calcium chloride dehydrate, magnesium chloride hexahydrate, cobalt chloride hexahydrate, and other metal salts. Methods of delivering a controlled release of surfactants composition, the method comprising the steps of: delivering a solution into a reservoir, the solution comprising an aqueous sulfonate solution; an anionic surfactant; and a salt selected from aluminum nitrate nanohydrate, calcium chloride dehydrate, magnesium chloride hexahydrate, cobalt chloride hexahydrate, and other metal salts; and delivering water to the reservoir.
Owner:SAUDI ARABIAN OIL CO
Improved drying agent
InactiveCN104258824ALow costWon't seepOther chemical processesDispersed particle separationSodium BentoniteCalcium Chloride Hexahydrate
The invention discloses an improved drying agent. The improved drying agent is prepared from the following raw materials in parts by weight: 4-8 parts of polyacrylamide, 5-20 parts of bentonite, 8-16 parts of silica gel, 3-7 parts of plant starch, 7-10 parts of calcium chloride dihydrate, 3-6 parts of lime powder, 2-6 parts of anhydrous calcium chloride, 3-15 parts of bamboo charcoal powder, 5-15 parts of refined iron powder, 5-10 parts of high water-absorbent resin, 6-11 parts of refined manganese dioxide, 5-10 parts of quick lime, 2-7 parts of calcium propionate, 7-13 parts of magnesium sulfate, 4-18 parts of attapulgite and 5-11 parts of calcium sulfate. The improved drying agent disclosed by the invention can be used for improving the moisture absorption capacity, is long in valid time and high in drying speed, and is not easy to cause a water return phenomenon.
Owner:QINGDAO HI TECH PATENT TECH TRANSFER PLATFORM
Technique for recycling triethylamine from hydrochloric acid triethylamine water solution
ActiveCN101293840ALow priceReduce dosageAmino compound purification/separationCalcium Chloride HexahydrateChloride
A method for recovering triethylamine from triethylamine hydrochloride aqueous solution comprises allowing calcium oxide and the triethylamine hydrochloride to react, and distilling at 50-150 DEC C to obtain the triethylamine; adding the calcium oxide into the collected triethylamine, refluxing under heating to remove water, and distilling to collect the triethylamine having water content smaller than 0.5%, distilling to obtain the triethylamine, separating solid from a turbid aqueous solution residual in a reaction kettle, adjusting the pH value of the aqueous solution to 7 with hydrochloric acid, and removing water to respectively obtain calcium chloride hexahydrate, calcium chloride dehydrate and anhydrous calcium chloride; and allowing the calcium oxide after drying the triethylamine and the triethylamine hydrochloride to react to obtain the triethylamine. Compared with the prior method for recovering the triethylamine from the triethylamine hydrochloride aqueous solution, the method has the advantages of simple operation, low price, high recovery rate, and no three wastes discharge.
Owner:凯米拉天成万丰化学品(兖州)有限公司
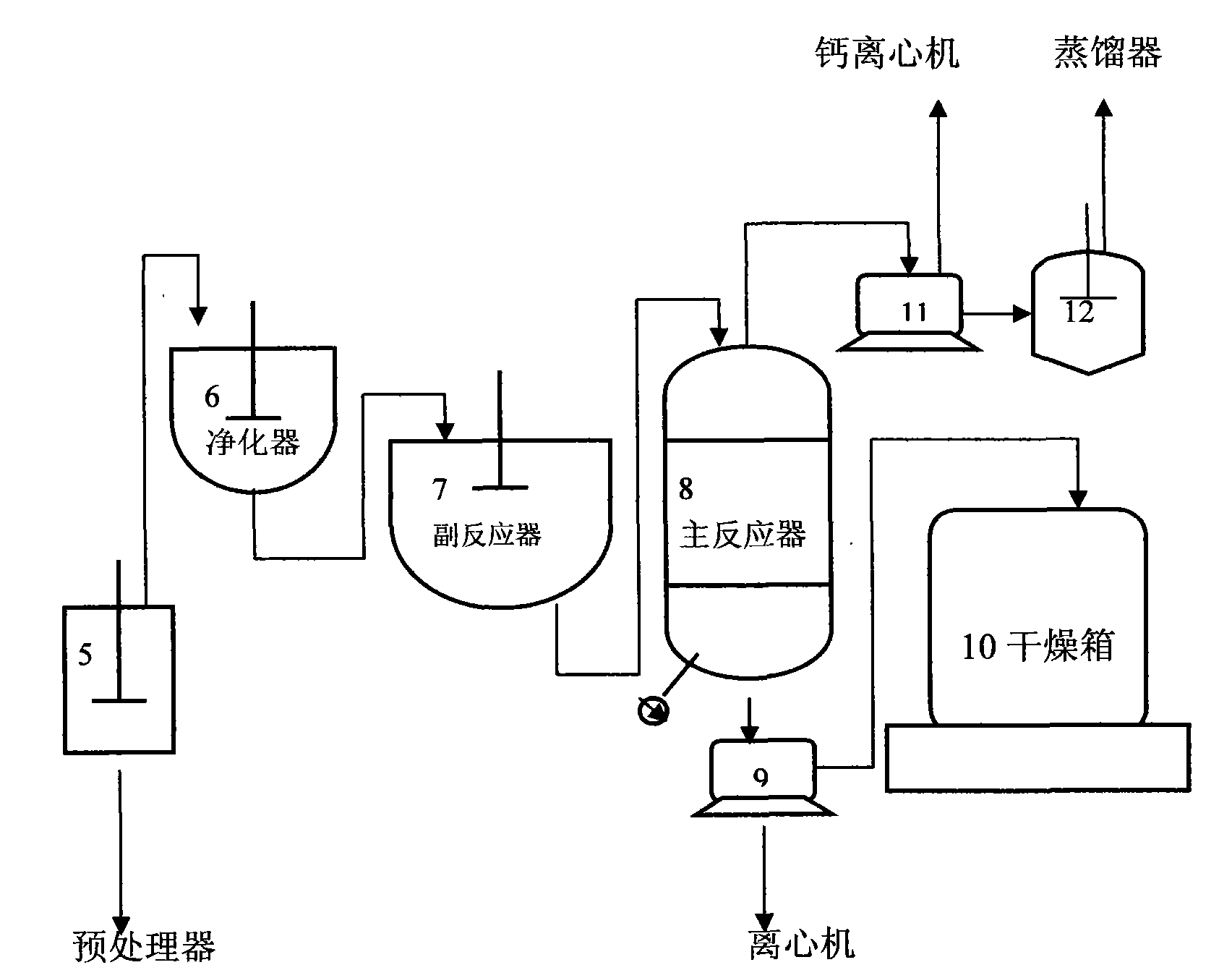
Method for producing calcium chloride dihydrate
InactiveCN101683997ANo pollution in the processHigh recovery rateCalcium/strontium/barium chloridesEmulsionCalcium Chloride Hexahydrate
The invention relates to a method for producing calcium chloride dihydrate, which is characterized by comprising the following steps: putting hydrochloric acid (31%) and limestone powder into a reaction vat according to the mixture ratio of 2.2:1, and stirring for reaction to generate an acid calcium chloride solution; transferring the obtained solution into a clarification tank, adding lime emulsion, regulating the pH value of the solution to 8.9-9 to precipitate out iron hydroxide and magnesium hydroxide; and transferring filtrate into an evaporation dish after clarifying and filtering, heating to the temperature of 172-174 DEG C, evaporating, drying and dewatering at the temperature of 200-240 DEG C after crystallizing and separating, to obtain the calcium chloride dihydrate. The invention has the following advantages of no pollution, high recovery rate and product cost reduction.
Owner:TIANJIN LIHONG CHEM TECHN
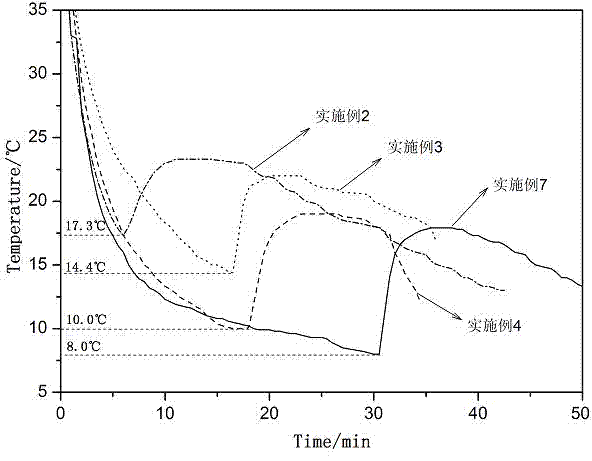
Low-temperature calcium chloride hexahydrate heat-storage material and preparation method
InactiveCN103923613AHigh phase change enthalpyPhase transition temperature is suitableHeat-exchange elementsCelluloseCalcium Chloride Hexahydrate
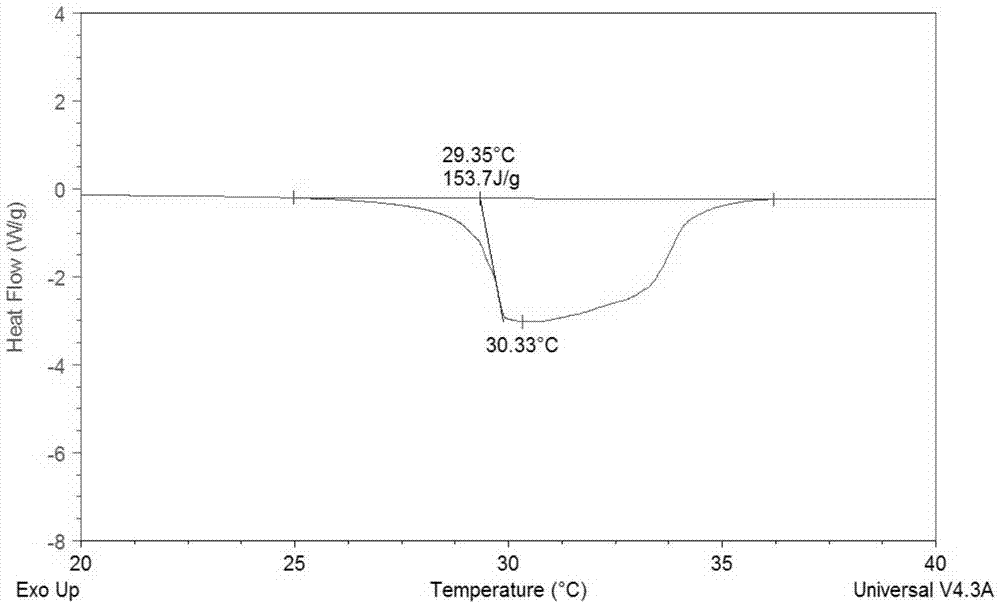
The invention discloses a low-temperature calcium chloride hexahydrate heat-storage material and a preparation method. The prepared low-temperature calcium chloride hexahydrate phase-change heat-storage material comprises a nucleating agent and a thickener according to mass percent, wherein the nucleating agent is borax, alumina or sodium metasilicate nonahydrate, the thickener is sodium carboxymethl cellulose (CMC), wherein calcium chloride hexahydrate is a phase-change base material, and the usage amount of the calcium chloride hexahydrate is 95%-98%; the preparation method comprises the step of: respectively adding 1wt% of borax and 1% of CMC or 1% alumina and 4% of CMC or 1% of sodium metasilicate nonahydrate and 2% CMC for modifying the calcium chloride hexahydrate, to obtain the low-temperature calcium chloride hexahydrate heat-storage material. The phase-change latent heat of the low-temperature calcium chloride hexahydrate heat-storage material is about 150J / g, the phase-change temperature of the low-temperature calcium chloride hexahydrate heat-storage material is at 25-30DEG C, the cooling degree is less than 2DEG C, and the heat suction and release performances of the low-temperature calcium chloride hexahydrate heat-storage material after being circulated for 3000 times are stable. The low-temperature calcium chloride hexahydrate heat-storage material has excellent application prospects in agricultural facilities and residential housing.
Owner:NORTHWEST A & F UNIV
Novel purification method for formic acid
ActiveCN101391945AOperating temperature requirements are not highReduce energy consumptionCarboxylic compound separation/purificationHigh concentrationPurification methods
Owner:ZHEJIANG UNIV OF TECH
Synthesis method of light stabilizer HS-112
InactiveCN106699639AReduce generationThe reaction steps are simpleOrganic chemistryChemical synthesisCalcium Chloride Hexahydrate
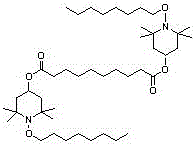
The invention discloses a synthesis method the a light stabilizer bis(1-octyoxy-2,2,6,6-tetramethyl-4-piperidyl) sebacate, belongs to the field of organic chemical synthesis. The bis(1-octyoxy-2,2,6,6-tetramethyl-4-piperidyl) sebacate is the material, ethanol solution is the solvent, and magnesium hydrate or magnesium chloride hexahydrate is the catalyst. Oxidation reaction is conducted with the action of aquae hydrogenii dioxidi which is used as the oxidant to generate bis(2,2,6,6-tetramethyl-1-piperidinyloxy-4-yl)sebacate free radicals. Then molybdenum trioxide is used as the catalyst, normal octane is used as the material and solvent to react with the bis(2,2,6,6-tetramethyl-1-piperidinyloxy-4-yl)sebacate free radicals to obtain the light stabilizer HS-112. The method uses two steps to synthesize the HS-112. The process is simple, the reaction time is short the side effects are few, the pollution is minimal, the production cost is low, the reaction yield rate is high, and therefore the industrial application prospect is good.
Owner:GANSU RES INSTION OF CHEM IND GRICI
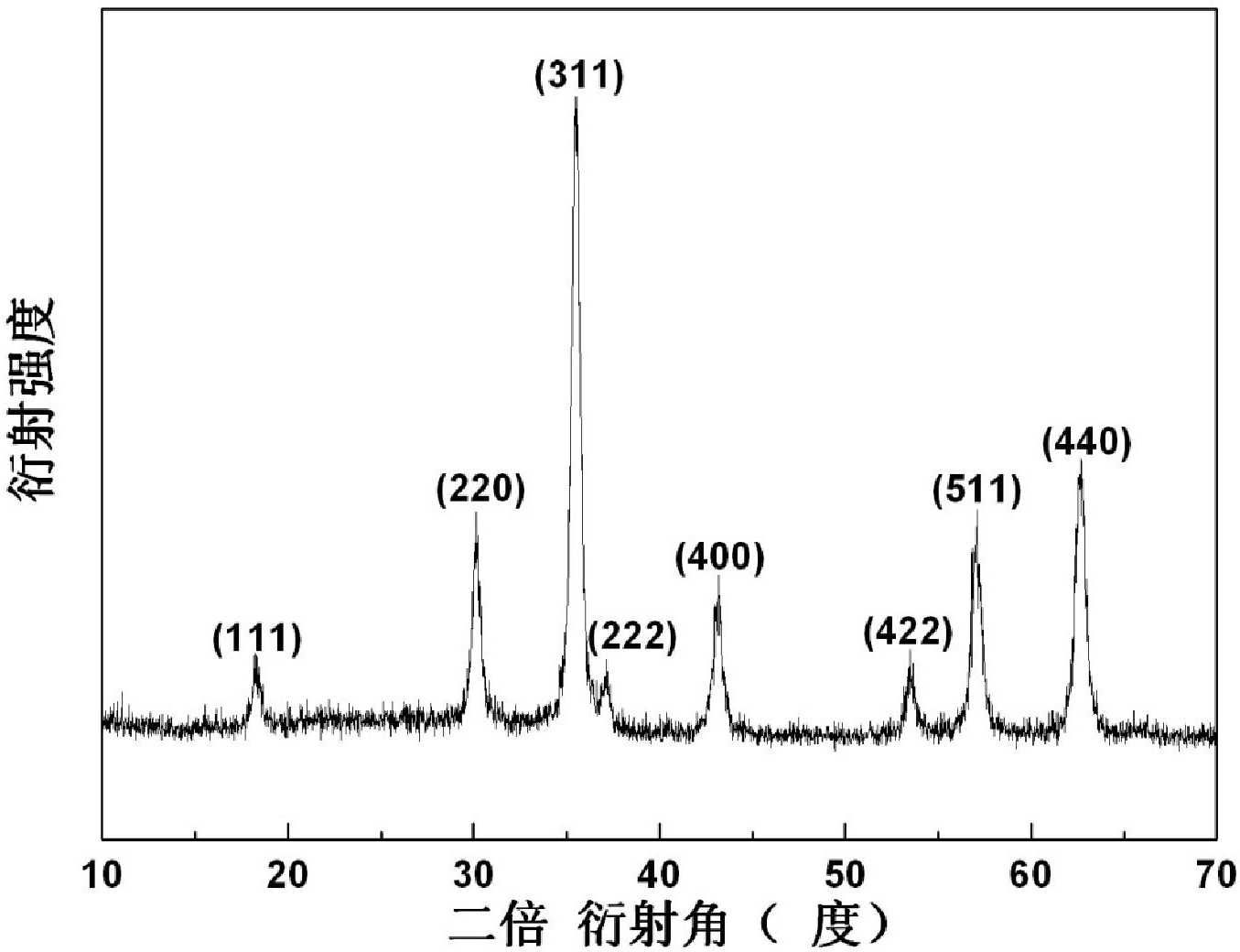
Hydrothermal preparation method for spinel type ferrite nanopowder
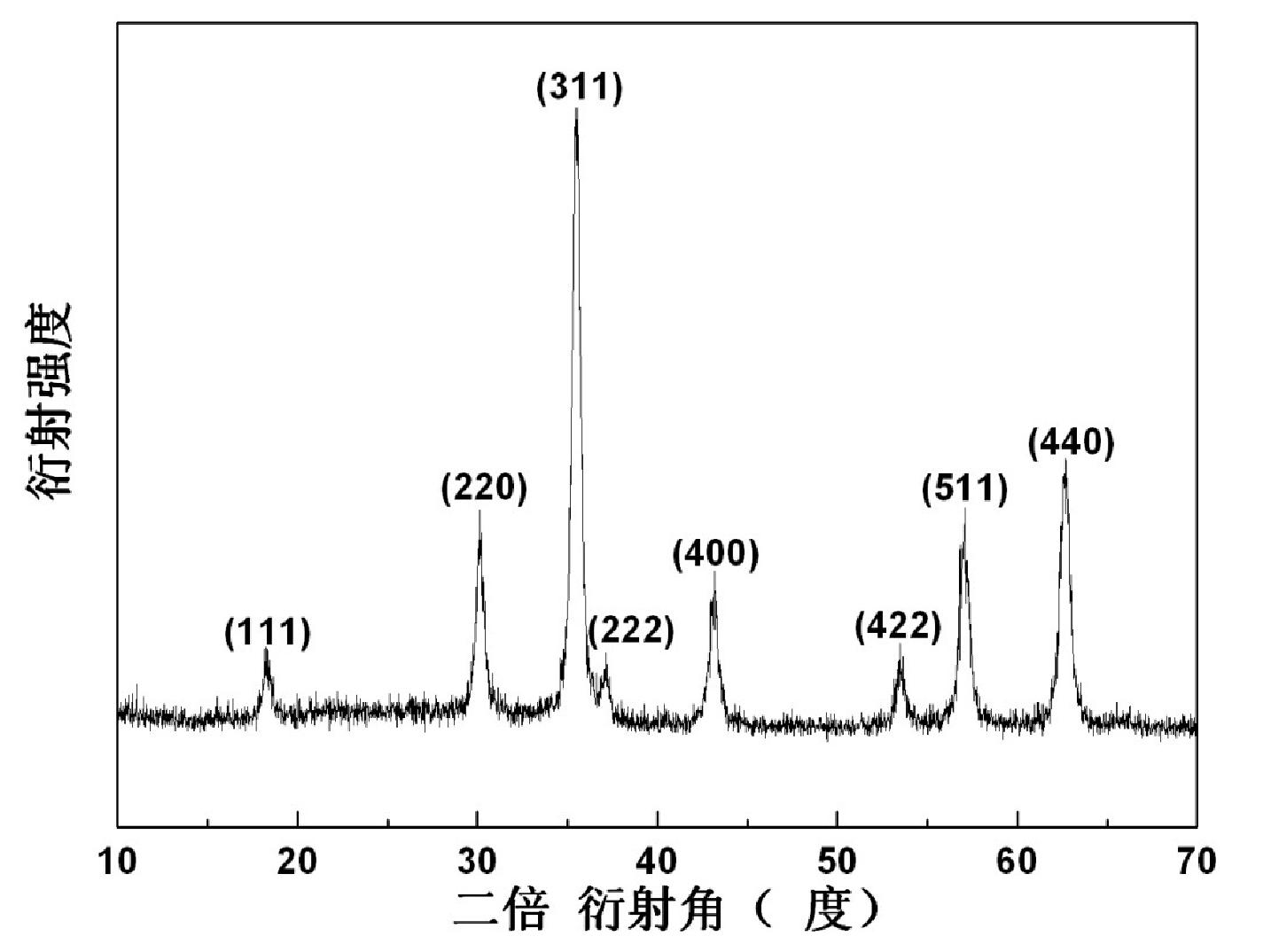
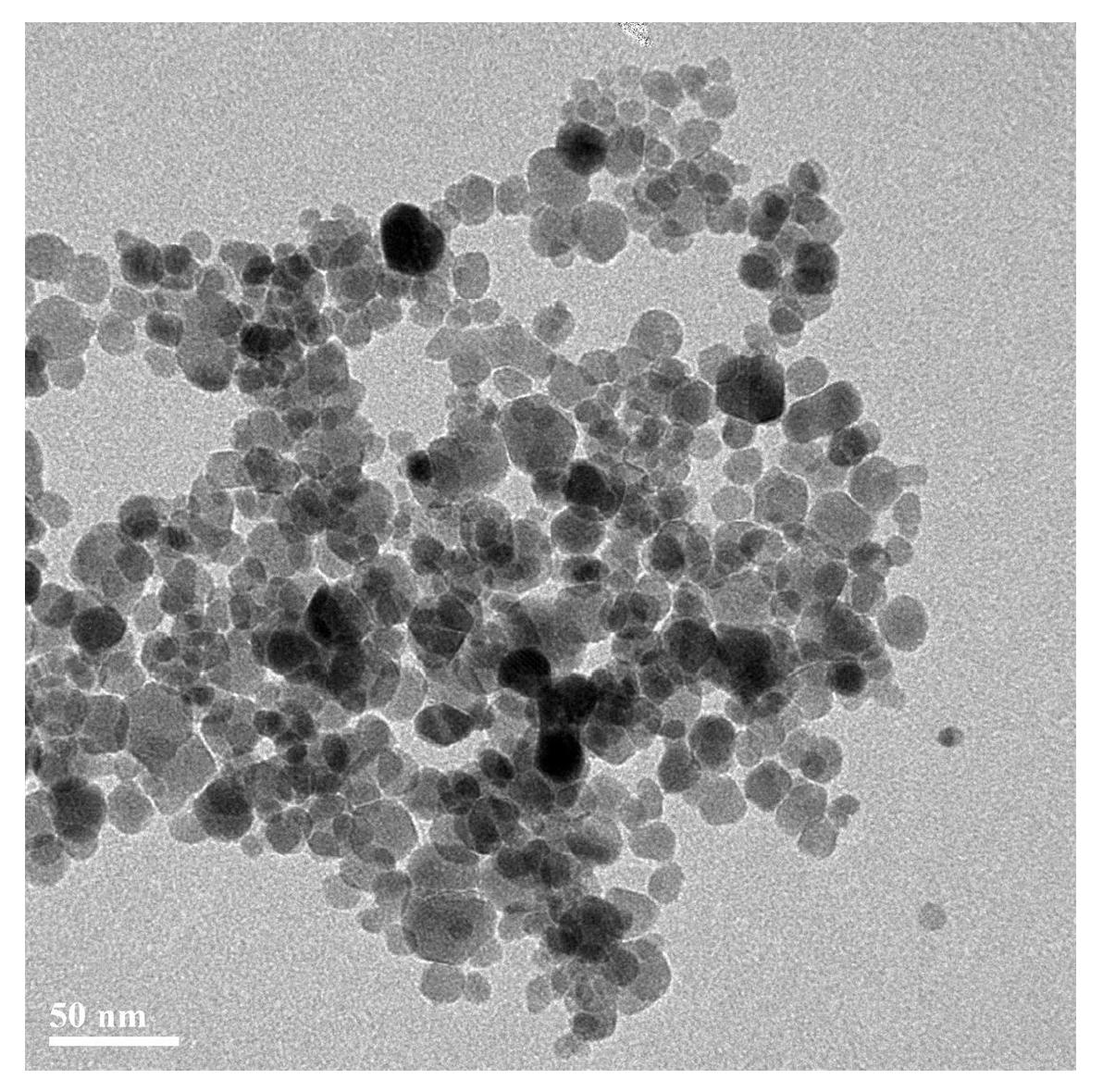
The invention relates to a hydrothermal preparation method for spinel type ferrite nanopowder. According to the hydrothermal preparation method, zinc nitrate hexahydrate [Zn(NO3)3 6H2O], cobalt nitrate hexahydrate [Co(NO3)2 6H2O], nickel chloride hexahydrate [NiCl2 6H2O] and iron nitrate nonahydrate [Fe(NO3)3 9H2O)] are selected as raw materials and ammonia water [NH3 H2O] and ethanolamine [C2H7NO] are selected as mineralizing agents. According to the hydrothermal preparation method, the ammonia water and the ethanolamine are used as the mineralizing agents and particles of the prepared material are spherical, uniform in size about a few tens of nanometers and favorable in dispersibility. The hydrothermal preparation method is simple in operation and low in cost and has important significance for spinel type ferrite as an important industrial magnetic material and catalyst with excellent performances, which is widely applied to electronics, communication and chemical industries.
Owner:SHANGHAI TITANOS IND
Phase change energy storage material for heat preservation at night and preparation method
InactiveCN103205242ALower eutectic pointLower melting temperatureHeat-exchange elementsCalcium Chloride HexahydrateMagnesium chloride hexahydrate
The invention relates to a phase change energy storage material for heat preservation at night. The medium consists of a CaC12-MgCl2-H2O water salt system and a nucleating agent; the CaC12-MgCl2-H2O water salt system consists of 80-95 % of calcium chloride hexahydrate, 1-7 % of magnesium chloride hexahydrate and 3-15 % of deionized water by mass; and the content of the nucleating agent is 0.5-2.0 % of the total amount of the CaC12-MgCl2-H2O water salt system. The phase change energy storage material has the advantages of high phase change latent heat, relatively low melting temperature and adjustable solidification temperature, can be used for avoiding the phase separation phenomenon, and can be applied to different seasons and different regions.
Owner:QINGHAI INST OF SALT LAKES OF CHINESE ACAD OF SCI
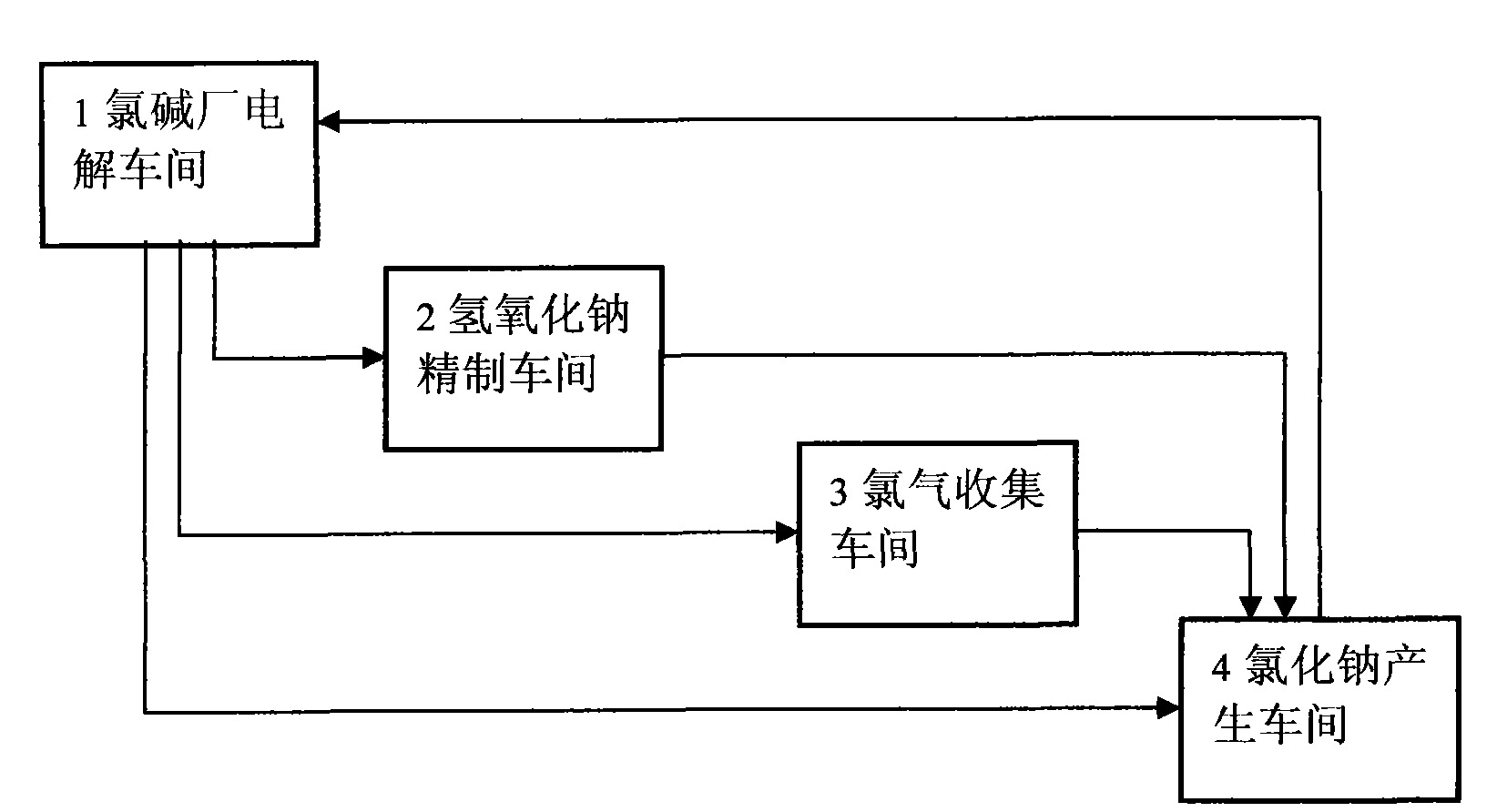
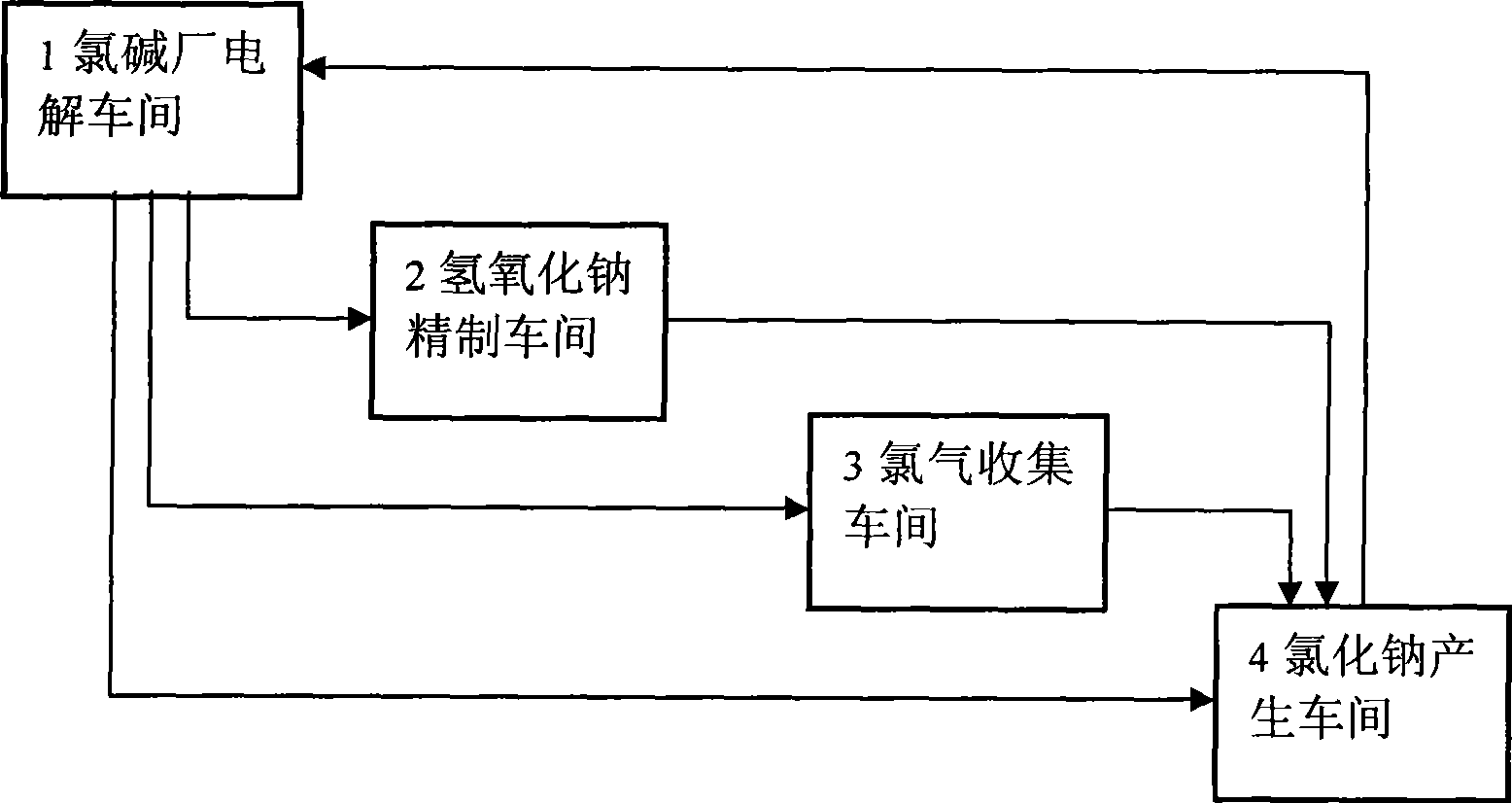
Material circulating system formed in chlor-alkali industry by using waste carbide slags and chlorine water
The invention discloses a material circulating system formed in the chlor-alkali industry by using waste carbide slags and chlorine water. The system is specifically as follows: after being preprocessed, the waste carbide slags react with hydrochloric acid and the reactant is filtered to obtain calcium chloride solution; the filtrate and sodium hydroxide generated in electrolysis in chlor-alkali plants generate high-purity calcium hydroxide; bleaching powder concentrate is produced after the calcium hydroxide is chloridized, centrifuged, dried and broken; filter cakes react with the hydrochloric acid to produce active carbon; calcium chloride generated in the reaction can be produced into calcium chloride dehydrate and calcium chloride anhydrous; after being processed, sodium chloride serves as a raw material for electrolysis. The system effectively solves the processing and disposal problems of the waste hydrated limes and the raw material problems in the chlor-alkali industry, embodies the circular economy, produces the byproducts including the bleaching powder concentrate and the active carbon and the products including the calcium chloride dehydrate and the calcium chloride anhydrous and improves the deep processing capabilities of the enterprises.
Owner:南通宙亚电子科技有限公司
Three-dimensional network-like chitosan-calcium carbonate nano composite material as well as preparation method and cell compatibility thereof
InactiveCN102343115ACompact structureEvenly distributedElectrolytic coatingsTissue/virus culture apparatusCalcium Chloride HexahydrateBiocompatibility Testing
The invention discloses a three-dimensional network-like chitosan-calcium carbonate nano composite material as well as a preparation method and cell compatibility thereof. In the preparation method, the chitosan-calcium carbonate nano composite material is prepared by taking chitosan as a carbon source, calcium chloride dehydrate, ammonium bicarbonate and secondary distilled water as raw materials on the surface of stainless steel by virtue of a one-step coelectrodeposition method. The biocompatibility of the chitosan-calcium carbonate network-like nano composite structure is studied by virtue of in-vitro cell culture. The electrodeposition method is simple and controllable; and the chitosan-calcium carbonate fibrous network-like structure is firmly combined with a substrate, and has strong mechanical performance and excellent biocompatibility. A good experimental result is achieved by using the chitosan-calcium carbonate fibrous network-like structure as a cytoskeleton material. The preparation method is simple in process, low in cost and environmentally-friendly; and the three-dimensional network-like chitosan-calcium carbonate nano composite material is in accordance with the standard of a biomaterial of a cell culture medium in tissue engineering, and can be applied to bone repair.
Owner:HUAZHONG NORMAL UNIV
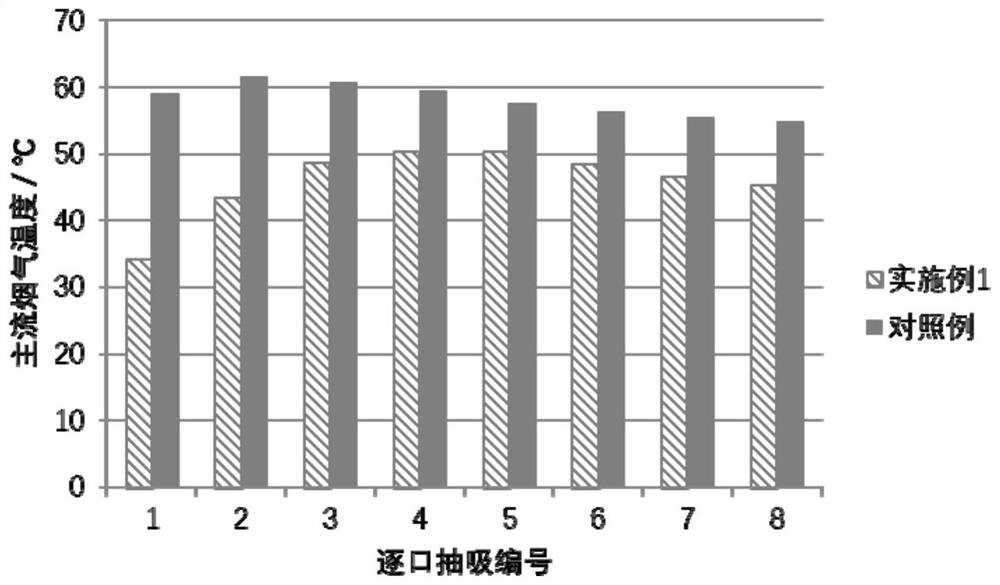
Cigarette cooling gel particles and application thereof
ActiveCN111602845AImprove the heat absorption effectSolve the leakTobacco smoke filtersPolyvinyl alcoholCalcium Chloride Hexahydrate
The invention discloses cigarette cooling gel particles and application thereof. The cigarette cooling gel particles comprise a base material and a water-soluble phase-change material, the water-soluble phase-change material is immobilized on the base material, the base material comprises polyvinyl alcohol, and the water-soluble phase-change material comprises one or more of polyethylene glycol, sugar alcohol, sodium sulfate decahydrate, calcium chloride hexahydrate and potassium chloride. The water-soluble phase-change material is immobilized in the base material, so that the problem of leakage of the phase-change material in the phase conversion process in the prior art is solved. Besides, the polyvinyl alcohol in the base material can be converted from a glassy state to a high-elastic state in a smoke temperature interval and can absorb a certain amount of heat, so that the overall heat absorption capacity of the cigarette cooling gel particles is further improved.
Owner:SHENZHEN TOBACCO IND
Highly effective fire-proof dust-suppressing material and its synthesis method
InactiveCN1884426ALow corrosion rateAvoid damageOther chemical processesDust removalSynthesis methodsCalcium Chloride Hexahydrate
The invention relates highly effective fireproof dust suppression material and method. The material comprises 60% calcium chloride dehydrate, 20% magnesium chloride hexahydrate, 20% water and surface active agent. The surface active agent comprises wetting agent, dispersing agent and inhibitor. The calcium chloride-dehydrate and magnesium chloride hexahydrate have hydroscopicity and fire-retardancy, and the surface active agent has the advantages of reducing surface tension, humectation and thickening action. The invention has the advantage of reducing corrosion. It can be used in anti-dust, fireproof fields.
Owner:SHANGHAI DATUN ENERGY
Treatment method of hydrochloric acid waste liquid from steel pickling
InactiveCN104529032AEnable recyclingSuitable for development needsWater treatment parameter controlWaste water treatment from metallurgical processLiquid wasteSludge
The invention relates to the technical field of steel pickling waste liquid, and especially relates to a treatment method of hydrochloric acid waste liquid from steel pickling. The iron grade in the treated waste residue reaches 44-50% and can meet the requirements of steel mills on the iron grade quality, and a calcium chloride solution can meet the requirements of other enterprises. Zero pollution emission after hydrochloric acid waste liquid treatment is achieved, and resources are recycled. An oxidation neutralization method is improved and upgraded to enable liquid calcium, calcium chloride dihydrate, anhydrous calcium chloride and sludge produced after the treatment to meet the standard requirements and the industrial demands, so as to recycle the resources. The project investment is little, the treated products can be used in industrial production to both achieve zero emission of treated pollution wastes and recycle the resources, and moreover the operation cost is low, so that the treatment method is suitable for the development demands of small and medium-sized enterprises.
Owner:胡志刚
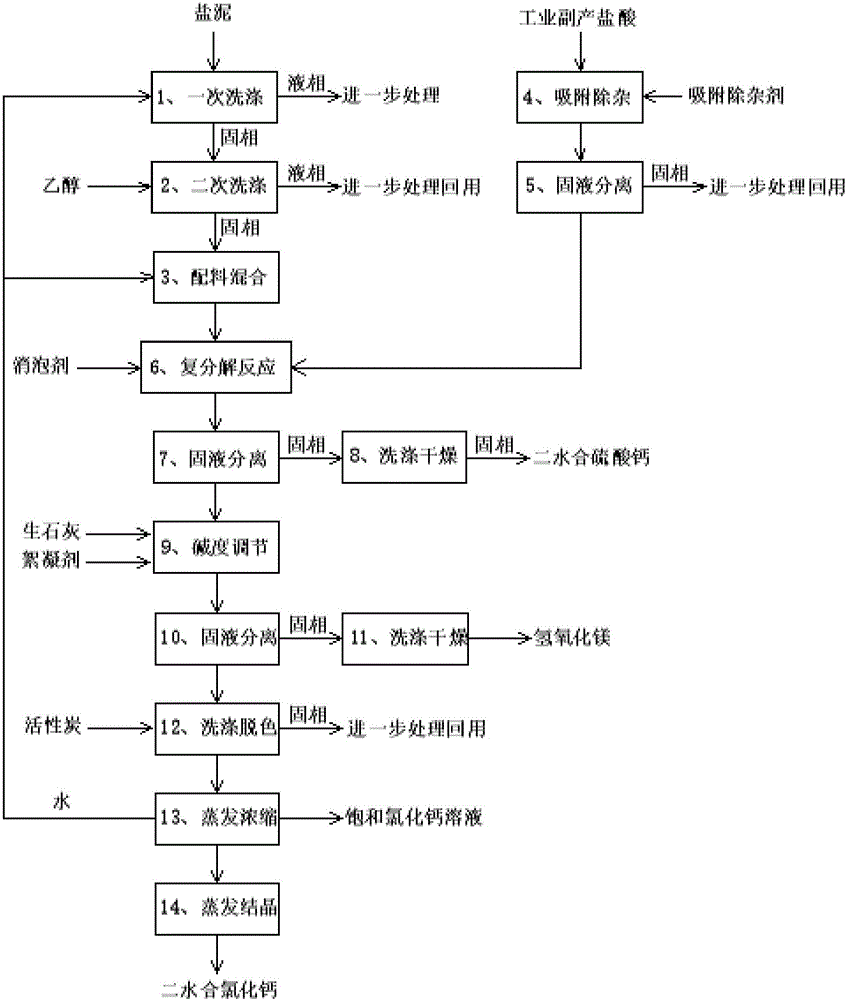
Resource comprehensive utilization for salty mud
InactiveCN105923646AReduce consumptionIn line with energy saving and emission reductionCalcium/strontium/barium chloridesCalcium/strontium/barium sulfatesCalcium Chloride HexahydrateSulfate
The invention relates to the technical field of salty mud utilization, in particular to a resource comprehensive utilization for salty mud. The salty mud and industrial by-product hydrochloric acid react and are separated to prepare calcium sulfate dihydrate, magnesium hydrate and calcium chloride dehydrate, solid waste is cyclically used to consume the salty mud well, and the product calcium chloride is widely used as a drying agent, a snow melting agent, a refrigerant, an antifreezing agent, coagulator and chemical raw materials. Separated calcium sulfate can be used for preparing a gypsum board and other building materials, and magnesium hydrate is good fire retardant for plastic and rubber products. Undoubtedly, comprehensive utilization of the salty mud and industrial by-product hydrochloric acid meets the policies and guidelines of energy conservation, emission reduction and environmental protection in China.
Owner:CHINASALT JINTAN
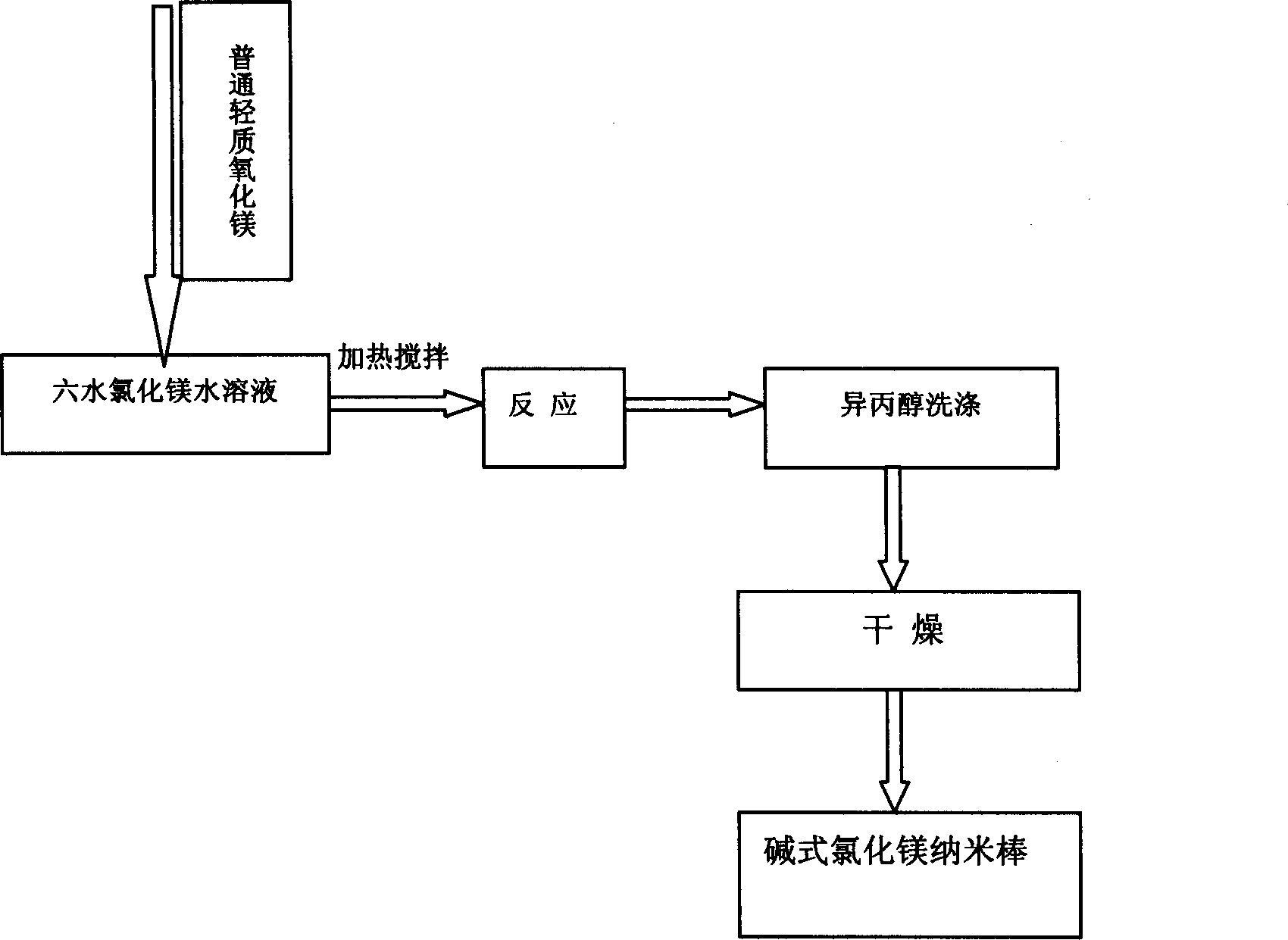
Liquid phase chemical reaction method for preparing basic magnesium chloride
InactiveCN1769178ALower operating temperatureReduce operating energy consumptionMagnesium chloridesChemical reactionAlcohol
The invention belongs to the art of preparation of one-dimensional nanometer material. It mainly contains: using magnesium chloride hexahydrate and common light magnesium oxide as raw material, firstly preparing gel-state basic magnesium chloride deposit, then filtering out the deposit and washing with deionized water, washing deposit with alcohol isopropylicum, filtering and drying to prepare basic magnesium chloride nano-stick of diameter of about 100-200nm and of length of about 4-8um. The basic magnesium chloride nano-stick is characterized in that the whiteness is good, the activity is strong and it is hyperfine, and it has broad utility prospect in the field of flame retardancy, paper making, fire-fighting, thermosol filler, and so on. And the merit of the process is characterized in that the operation temperature is low, the technique is simple, the production cost is low, the yield is high, it is easy for industrial production, and so on.
Owner:DALIAN UNIV OF TECH
Material circulation system formed by waste carbide mud residue and alkali-chloride industry
InactiveCN101456026ASolve disposal problemsSolve the problem of raw materialsElectrolysis componentsSolid waste disposalElectrolysisCalcium Chloride Hexahydrate
The invention discloses a material circulating system formed by waste acetylene sludge and the chlor-alkali industry. The system comprises: pretreated waste acetylene sludge is reacted with hydrochloric acid, the reaction product is filtered to obtain a calcium chloride solution; the filtrate is reacted with sodium hydroxide generated by electrolysis of a chlor-alkali factory to generate high-purity calvital; the calvital is chloridized, centrifugated, dried and crushed to prepare bleaching powder; and filter cakes are reacted with the hydrochloric acid to generate active carbon. The calcium chloride generated in the reaction can be prepared into calcium chloride dihydrate and anhydrous calcium chloride; and sodium chloride after treatment can be taken as a raw material for electrolysis. The material circulating system effectively solves the problem of treating the waste acetylene sludge and raw material problem in the chlor-alkali industry, embodies recycling economy; and byproducts of the bleaching powder and the active carbon and calcium chloride dihydrate products improve deep processing capacity of an enterprise.
Owner:南通宙亚电子科技有限公司
Rare earth yttrium doped molybdenum disulfide self-lubricating composite coating and preparation method thereof
ActiveCN108149220AHigh purityLow content of impurity elementsChemical vapor deposition coatingCalcium Chloride HexahydrateGas phase
The invention provides a rare earth yttrium doped molybdenum disulfide self-lubricating composite coating and a preparation method thereof. Specifically, the molybdenum disulfide self-lubricating composite coating is doped with rare earth yttrium, wherein the molar content of yttrium in the composite coating is 0.1%-5% according to the total molar content of the composite coating. According to themolybdenum disulfide self-lubricating composite coating, sublimed sulfur, yttrium chloride hexahydrate and molybdenum trioxide are adopted as raw materials, and the rare earth yttrium doped molybdenum disulfide self-lubricating composite coating is prepared through the chemical vapor deposition method. The molybdenum disulfide self-lubricating composite coating has good wear resistance and self-lubrication and excellent winding plating property. Coating deposition can be conducted on large-size complex workpieces of a complex shape.
Owner:NINGBO INST OF MATERIALS TECH & ENG CHINESE ACADEMY OF SCI
Phase-change gel heat-preservation material and preparation method thereof
InactiveCN107488020AWith insulationWith energy storageCalcium Chloride HexahydratePolyethylene glycol
The invention discloses a phase-change gel heat-preservation material and a preparation method thereof. The heat-preservation material comprises the following components in parts by weight: 23-48 parts of magnesium oxide, 12-35 parts of magnesium sulfate heptahydrate, 3-11 parts of paraffin, 2-10 parts of polyethylene glycol, 4-12 parts of sodium sulfafe decahydrate, 5-9 parts of calcium chloride hexahydrate, 3-9 parts of sodium carbonate decahydrate, 2-12 parts of aluminum hydroxide, 1-8 parts of hydrogen peroxide, 3-15 parts of a polypropylene fiber and 4-13 parts of a glass fiber. The phase-change gel heat-preservation material disclosed by the invention has excellent properties of heat preservation, energy storage and light weight and is high in strength and good in anti-cracking property.
Owner:苏州仲勉装饰有限公司
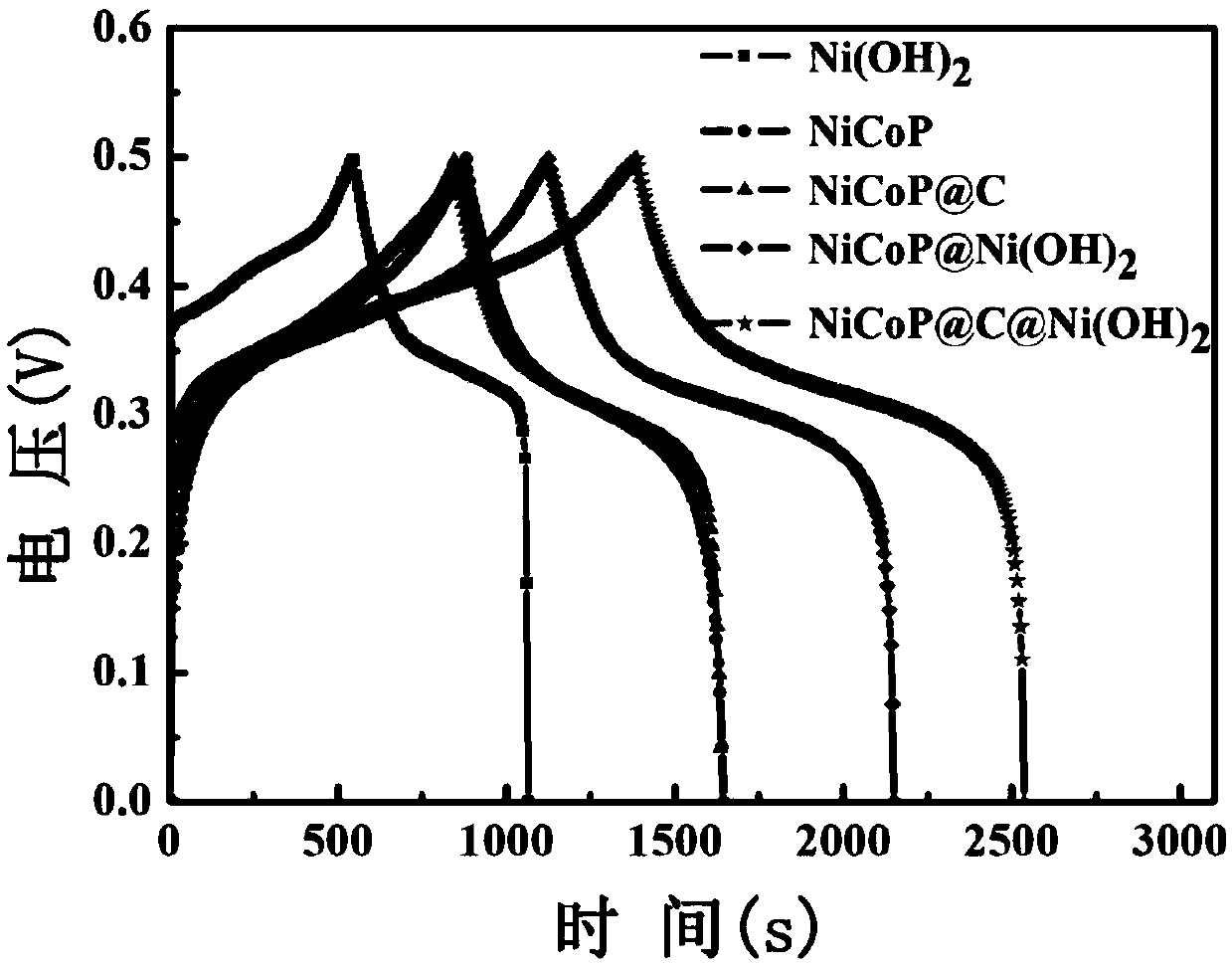
Preparation method of nickel-cobalt-phosphorus-carbon-nickel hydroxide ternary composite electrode material
ActiveCN109545576AImprove mechanical propertiesImprove cycle performanceMaterial nanotechnologyHybrid capacitor electrodesCapacitanceHigh energy
The invention, which relates to the scientific field of nano composite materials, aims at providing a preparation method of a nickel-cobalt-phosphorus-carbon-nickel hydroxide ternary composite electrode material. The method comprises the following steps: growing a nickel-cobalt sheet wire nano-array substrate on the surface of foamed nickel by using nickel nitrate hexahydrate, cobalt nitrate hexahydrate, ammonium fluoride and urea, carrying out immersion in a glucose solution and heat treatment in an argon atmosphere, and then carrying out reaction with sodium hypophosphite to obtain a nickel-cobalt-phosphorus-carbon nano array; and then making a reaction with nickel chloride hexahydrate and urea; to be specific, enabling the nickel chloride hexahydrate reacts with the urea to obtain foamed nickel loaded with a nickel-cobalt-phosphorus-carbon-nickel hydroxide nano-array. The preparation method has advantages of simple process and strong operability; the nanor array can grows directly on the foamed nickel substrate; and the prepared electrode can be used as the electrode of a super capacitor directly, so that the super capacitor has the broad application prospects. Therefore, the cycle performance, overall specific capacitance and energy density of the electrode material can be improved; and the thus the electrode has the higher energy density and excellent cycle performance.
Owner:ZHEJIANG UNIV
Anti-icing deicing asphalt based on phase-change energy storage exothermic material and preparation method thereof
ActiveCN105838096AAvoid damagePlay an active anti-icing and melting effectBuilding insulationsCalcium Chloride HexahydrateParaffin oils
The invention discloses anti-icing deicing asphalt based on phase-change energy storage exothermic material, made by mixing composite phase-change material and matrix asphalt, wherein the matrix asphalt accounts for 75-90% by weight and the composite phase-change material accounts for 10-25% by weight; the matrix asphalt is petroleum asphalt, oxidized asphalt, coal pitch, lake asphalt or rock asphalt; the composite phase-change material is gelatin powder and phase-change material composite made by mixing, according to a weight ratio of 75-80:20-25: sodium sulfate decahydrate, disodium hydrogen phosphate dodecahydrate, calcium chloride hexahydrate or paraffin, or their mixture of any weight ratio, and adsorbing material gelatin powder. The anti-icing deicing asphalt prepared herein is made from the gelatin powder supported phase-change material into phase-change material and gelatin powder composite for the purpose of energy storage and release, and anti-icing and deicing capacities are imparted to the asphalt itself; the preparation method of the asphalt is simple, the asphalt is free of negative impact on road surroundings and enables green road transportation.
Owner:SHANDONG JIAOTONG UNIV
Nickel-cobalt-iron ternary metal oxide nano tubular composite material and preparation method thereof
ActiveCN107195470AGood reversibilityImprove cycle stabilityHybrid capacitor electrodesHybrid/EDL manufactureWater bathsFiber
The invention discloses a nickel-cobalt-iron ternary metal oxide nano tubular composite material and a preparation method thereof. The preparation method comprises the steps of firstly, pre-treating and drying a carbon fiber fabric; preparing a mixed solution of cobalt chloride hexahydrate, nickel chloride hexahydrate, hexadecyl trimethyl ammonium bromide and urea by using distilled water as a solvent, putting the mixed solution and the carbon fiber fabric into a reactor for hydrothermal reaction, thus obtaining a NiCo2O4 precursor; and adding ferric chloride hexahydrate into the prepared precursor for water bath reaction, thus finally obtaining a NiCo2-xFexO4 nano tubular composite material. Because the prepared composite material has a tubular structure and is a nano particle, the specific surface area of the material is greatly increased, the electrode reaction kinetics is accelerated, and the super capacitive property of the material is improved. The composite material has excellent reversibility and cyclic stability. The preparation method is simple in operation and low in cost, and has a broad application prospect in the fields of energy storage, crystals and the like.
Owner:OCEAN UNIV OF CHINA
Preparation method of magnesium oxide board
ActiveCN107445581AReinforcing and tougheningHigh mechanical strengthCalcium Chloride HexahydratePolyethylene glycol
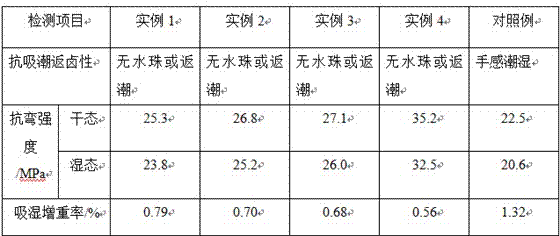
The invention discloses a preparation method of a magnesium oxide board, and belongs to the technical field of decoration materials of buildings. The preparation method comprises the following steps of uniformly stirring and mixing rice husk, quicklime and water, sealing and standing, and filtering, so as to obtain rice husk and quicklime mixed wet material; stirring light burning powder, hydrochloric acid and polyethylene glycol to react at constant temperature, filtering to obtain filtrate, and uniformly stirring and mixing with the light burning powder, magnesium chloride hexahydrate and the rice husk and quicklime mixed wet material, so as to obtain slurry; paving one layer of nonwoven cloth and one layer of magnesium oxide cloth at the bottom of a die, pouring the slurry into the die, forming, paving one layer of magnesium oxide cloth and one layer of nonwoven cloth, and drying, so as to obtain a blank of the magnesium oxide board; moving into a curing chamber to cure, fumigating by nitrogen carrying trimethylaluminum in the curing period, and discharging material after the curing is finished, so as to obtain the magnesium oxide board. The preparation method has the beneficial effects that the better property of resisting moisture absorbing and halogen reforming is realized; the bending strengths in dry and wet states are high; the weight increasing rate is low after moisture absorbing; the deformation of the magnesium oxide board in the use process is effectively avoided; the magnesium oxide board is suitable for being popularized and applied.
Owner:SHENZHEN HUAZHU HABITAT TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com