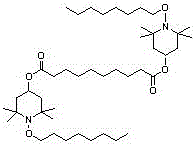
Synthesis method of light stabilizer HS-112
A technology of HS-112 and light stabilizer, which is applied in the direction of organic chemistry, can solve the problems of few research reports on synthetic methods, achieve good industrial application prospects, reduce side reactions, and simplify reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Weigh 60g of UV-770 dry product, 300mL of 95% ethanol, 2.0g of catalyst magnesium hydroxide, add them to a three-necked flask, stir and heat to 75℃, add hydrogen peroxide (30%H 2 O 2 , 56g), keep the reaction for 10h, the color of the reaction liquid gradually changes from colorless to red. After the reaction is over, filter out the catalyst while it is hot, and add appropriate amount of MnO 2 Remove excess hydrogen peroxide and filter out MnO 2 . Distill under reduced pressure at 50°C. Stop the distillation when no liquid flows out. At this time, the product in the flask is a viscous red substance—bis(2,2,6,6-tetramethylpiperidinyl)sebacate nitroxide Free radicals.
[0024] Add n-octane to the bis(2,2,6,6-tetramethylpiperidinyl) sebacate nitroxide radical to dissolve by heating, add molybdenum trioxide, heat up to 125°C, and add dropwise under reflux Tert-butyl hydroperoxide (TBHP, 60g), heat preservation and reaction for 3h, the reaction liquid gradually changes from re...
Embodiment 2
[0026] Weigh 60 g of UV-770 dry product, 300 mL of 95% ethanol, 2.0 g of magnesium chloride hexahydrate as a catalyst, add them to a three-necked flask, stir and heat to 75°C, add hydrogen peroxide (30% H 2 O 2 , 56g), keep the reaction for 10h, the color of the reaction liquid gradually changes from colorless to red. After the reaction is over, filter out the catalyst while it is hot, and add appropriate amount of MnO 2 Remove hydrogen peroxide, filter out MnO 2 . Distill under reduced pressure at 50°C. Stop the distillation when no liquid flows out. At this time, the product in the flask is a viscous red substance-bis(2,2,6,6-tetramethylpiperidinyl)sebacate nitroxide Free radicals.
[0027] Add n-octane to the bis(2,2,6,6-tetramethylpiperidinyl) sebacate nitroxide radical to dissolve by heating, add the catalyst molybdenum trioxide, heat up to 125℃, and add dropwise under reflux Tert-butyl hydroperoxide (TBHP, 60g), heat preservation and reaction for 3h, the reaction liquid gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com