Low-temperature calcium chloride hexahydrate heat-storage material and preparation method
A technology of calcium chloride hexahydrate and heat storage material, which is applied in the field of calcium chloride hexahydrate heat storage material and preparation, can solve the problems of insufficient heat release and supercooling, and achieves a suitable phase transition temperature and is simple and convenient to prepare. , the effect of easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of the above-mentioned low-temperature calcium chloride hexahydrate heat storage material uses calcium chloride hexahydrate as the phase change basic material, and adds a nucleating agent and a thickener therein for modification, including the following steps:
[0027] 1) Slowly add anhydrous calcium chloride into hot water at a constant temperature of 50°C until the anhydrous calcium chloride no longer dissolves and precipitates, then filter to obtain a saturated solution of pure calcium chloride;
[0028] 2) Put the pure calcium chloride saturated solution in a constant temperature environment at 5°C, cool down and crystallize the saturated calcium chloride solution to obtain calcium chloride hexahydrate crystals; place the calcium chloride hexahydrate crystals at a low temperature of 5°C to dry Low-temperature drying in the environment to obtain pure calcium chloride hexahydrate crystals;
[0029] 3) Add a nucleating agent and a thickener to pu...
Embodiment 1
[0033] In this example, anhydrous calcium chloride, borax and sodium carboxymethylcellulose (CMC) are used as raw materials to prepare low-temperature calcium chloride hexahydrate phase-change thermal storage materials, wherein the amount of anhydrous calcium chloride is 98% (mass ratio ), the amount of borax is 1% (mass ratio), and the amount of sodium carboxymethylcellulose is (CMC) 1% (mass ratio). First, pure calcium chloride hexahydrate crystals are obtained by dissolving and cooling crystallization. Add borax and sodium carboxymethylcellulose (CMC) to calcium chloride crystals. In this example, borax is used as a nucleating agent and sodium carboxymethylcellulose (CMC) is used as a thickener. Heat to 40°C until liquid, and stir uniform. The homogeneous solution is placed below 20°C for 24 hours to form a solidified calcium chloride hexahydrate thermal storage material.
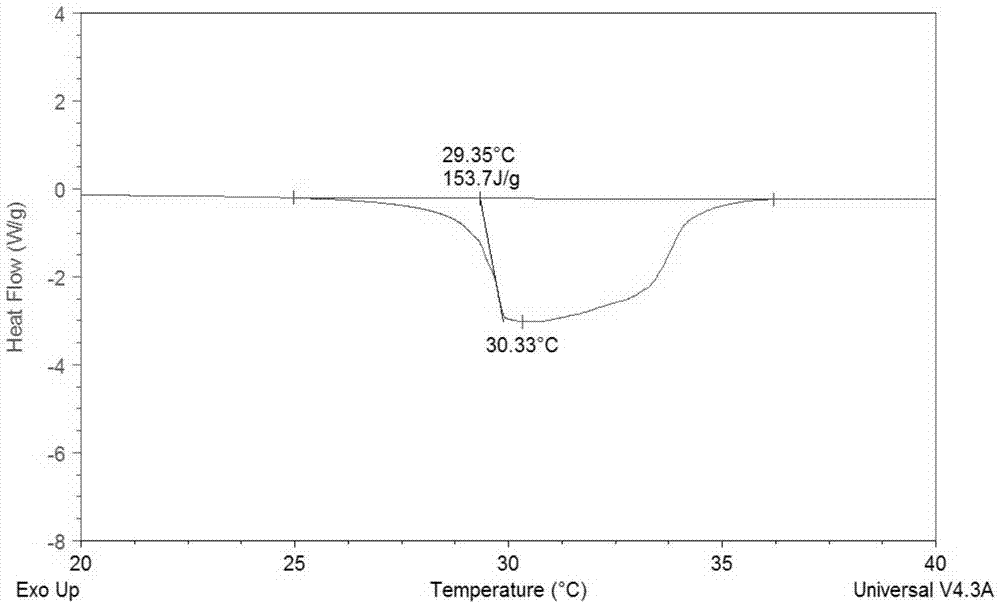
[0034] like figure 1 As shown, the test by the applicant shows that the low-temperature calcium chl...
Embodiment 2
[0036] This example also adopts the method of Example 1 to prepare low-temperature calcium chloride hexahydrate heat storage material, using anhydrous calcium chloride, aluminum oxide and sodium carboxymethylcellulose (CMC) as raw materials, wherein anhydrous calcium chloride The dosage is 95% (mass ratio), and the dosage of alumina is 1% (mass ratio). The amount of sodium carboxymethylcellulose (CMC) is 4% (mass ratio). In this example, aluminum oxide is used as a nucleating agent and sodium carboxymethylcellulose (CMC) is used as a thickener. First, it is obtained by dissolving and cooling crystallization. Pure calcium chloride hexahydrate crystals, add aluminum oxide and sodium carboxymethylcellulose (CMC) to pure calcium chloride hexahydrate crystals, heat to 40°C until liquid, and stir evenly. Put the homogeneous solution below 20°C for 24 hours to form a solidified calcium chloride hexahydrate heat storage material. The coldness is less than 2°C and there is no phase se...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
| melt | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com