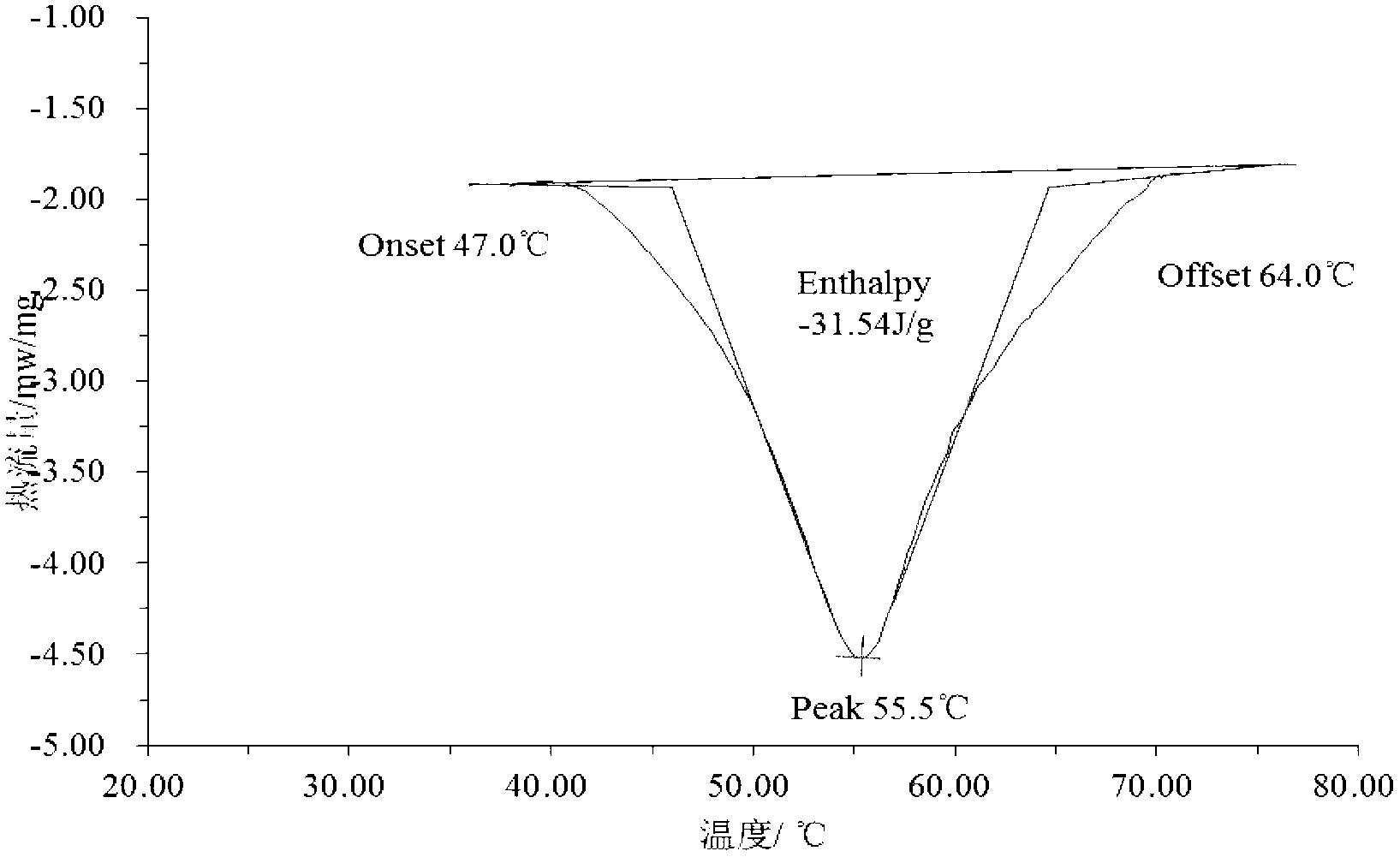
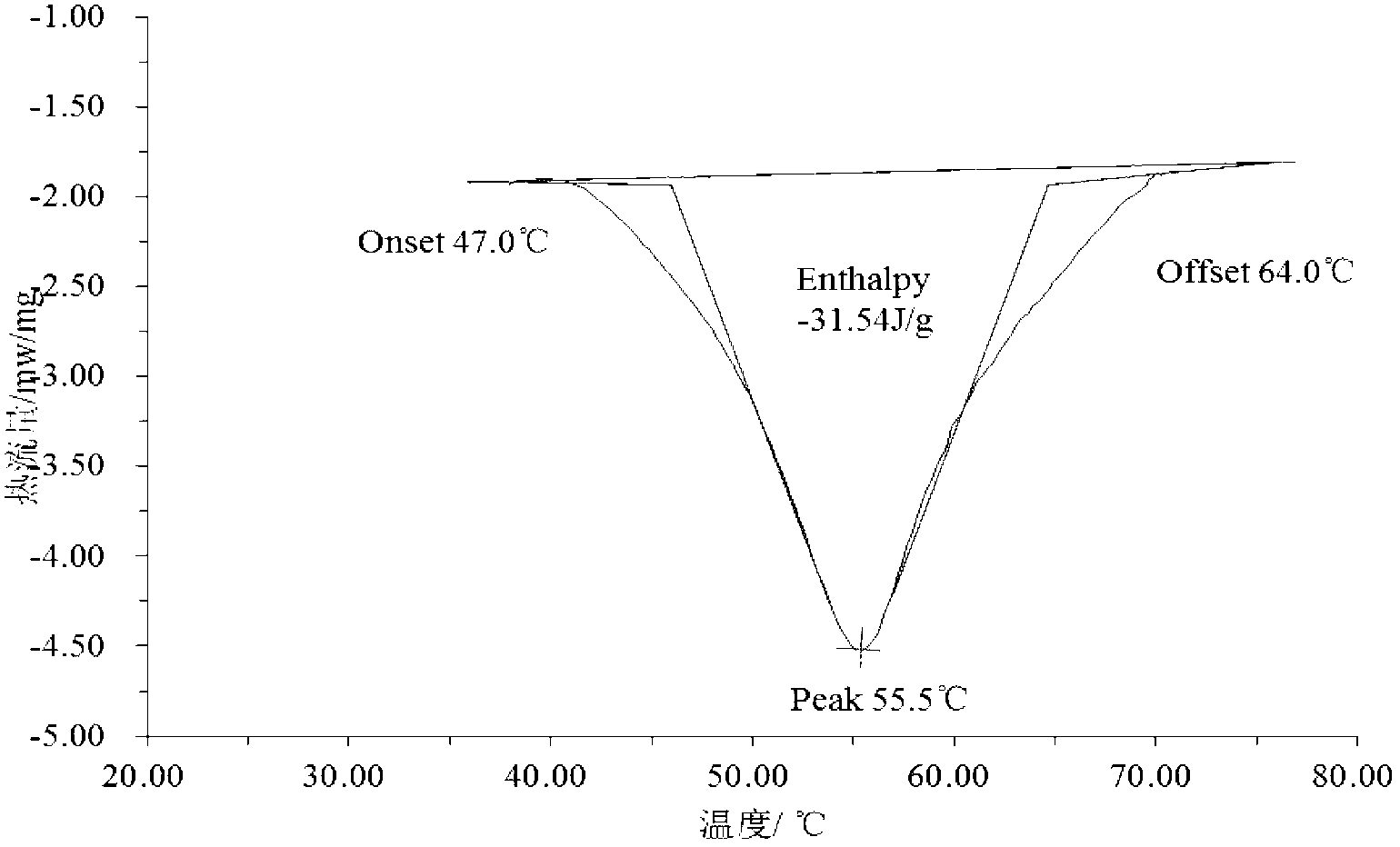
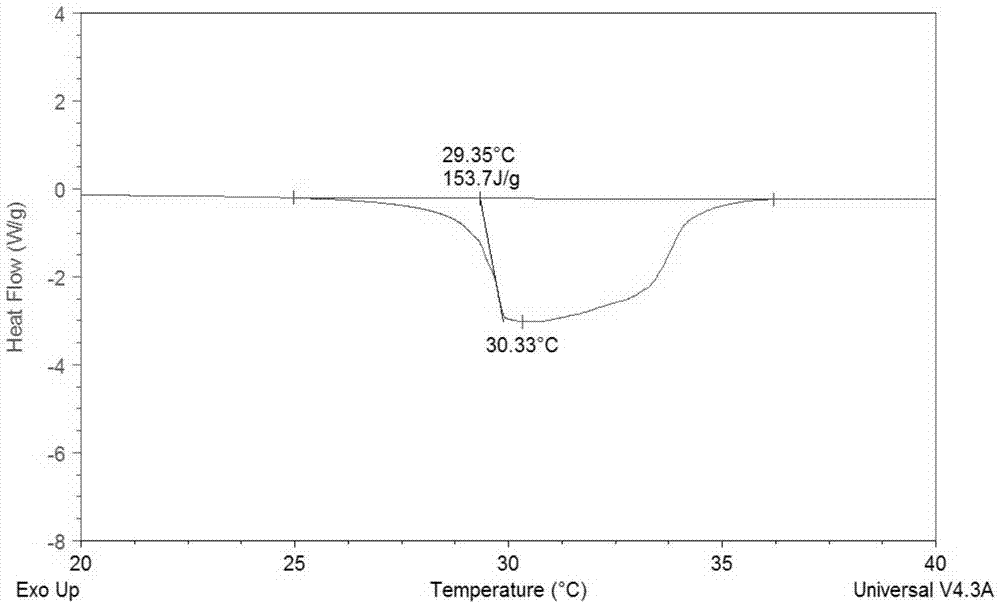
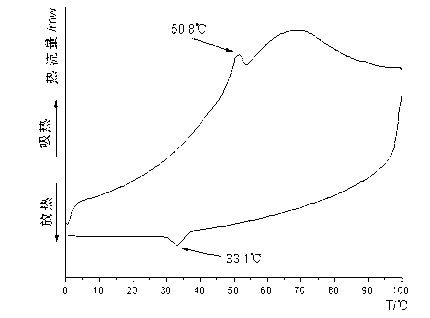
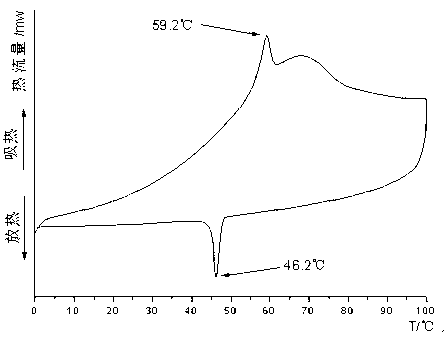
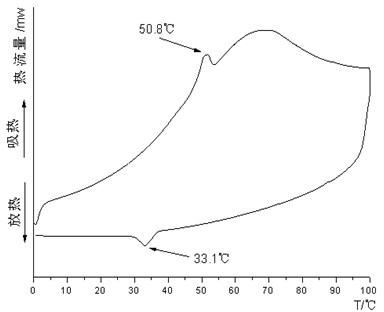
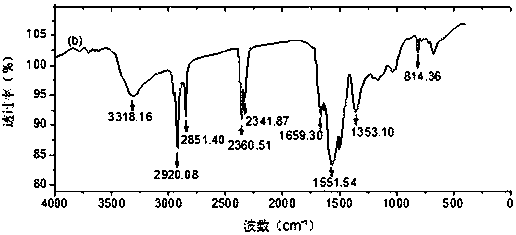
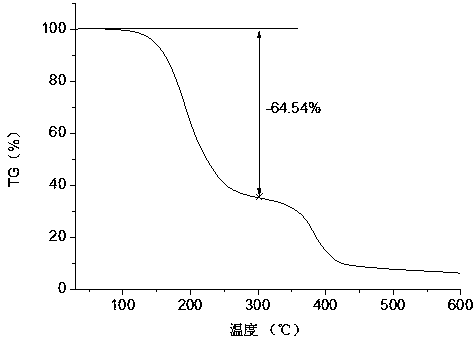
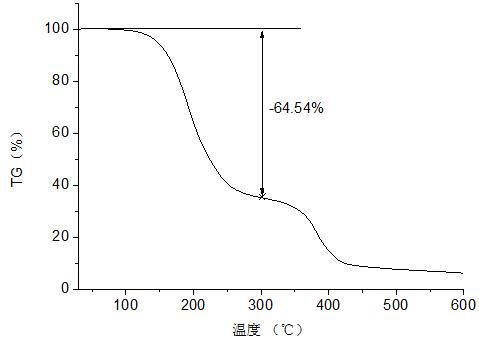
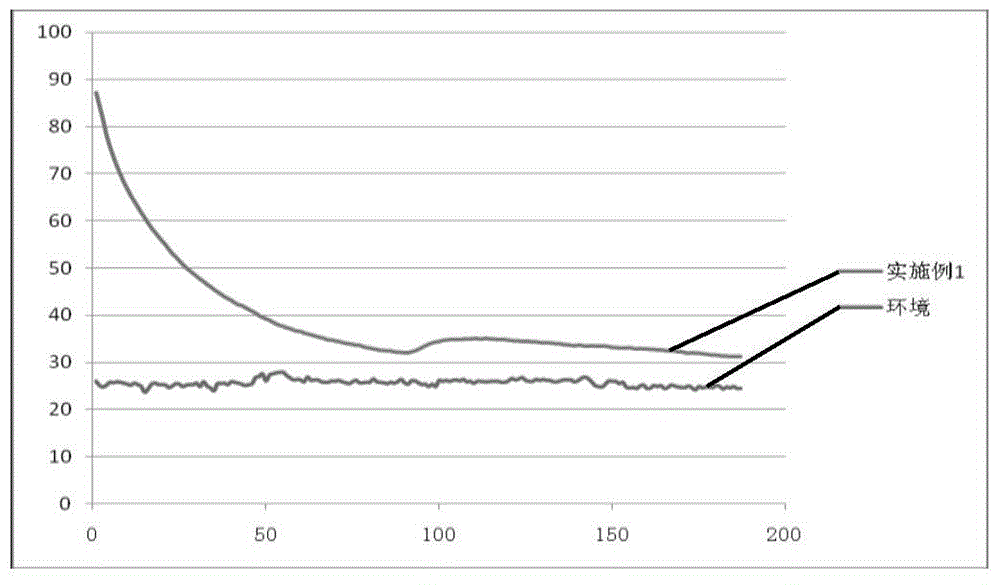
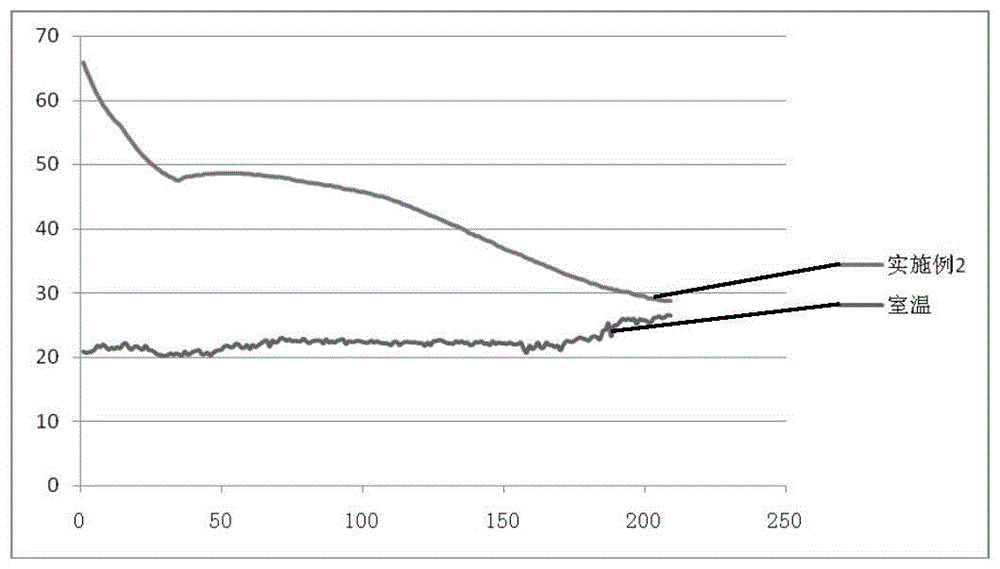
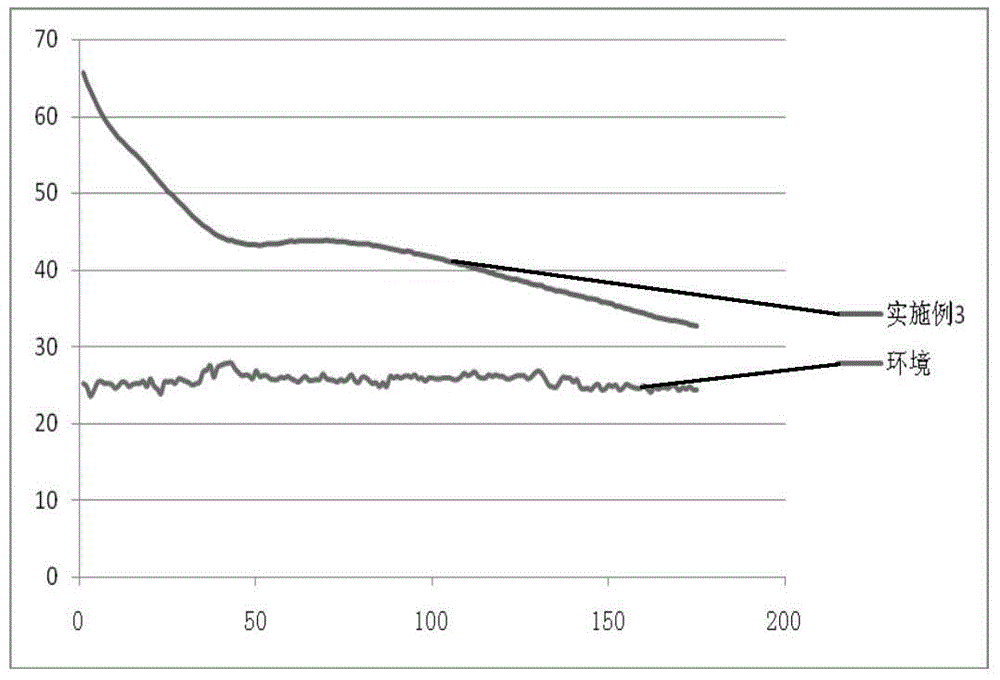
Patents
Literature
34results about How to "The phase transition process is reversible" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
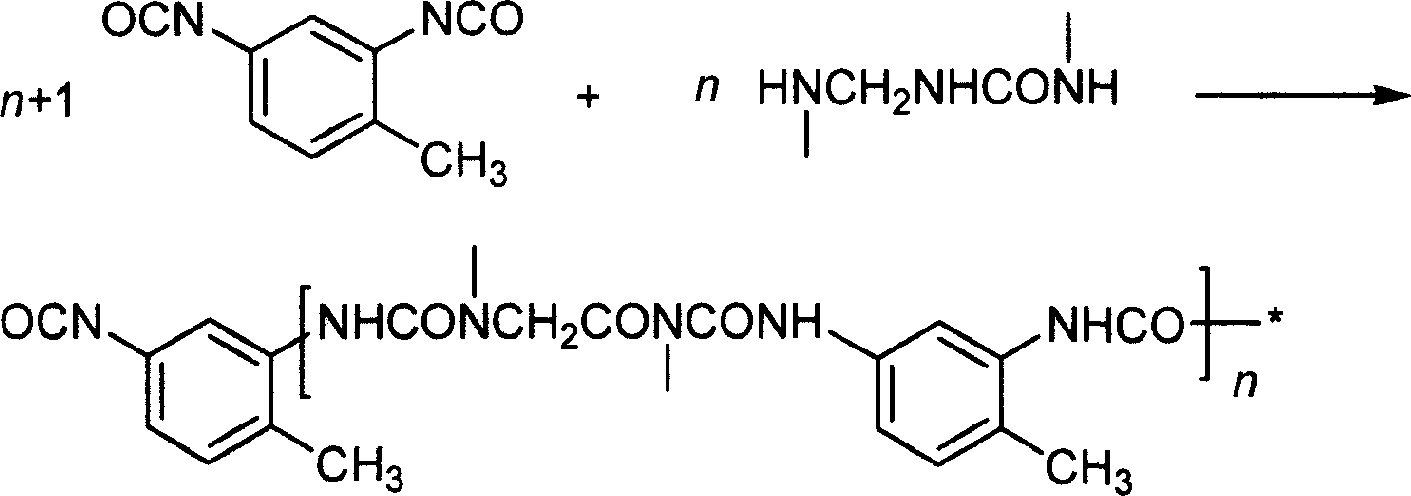
Preparation method of phase transformation material microcupsule
InactiveCN101045857AHigh mechanical strengthRaw materials are easy to getHeat-exchange elementsMicroballoon preparationParaffin waxToluene diisocyanate
This invention relates to preparation method of a facies change stuff microcapsule. It takes paraffin as phase-change material, takes toluene diisocyanate and urea-formaldehyde resin as cyst wall stuff, by interfacial polymerization and in situ polymeric method to carry out microencapsulate, to prepare double deck, microcapsule packed paraffin phase-change material. This method could prepare microcapsule phase-change material that contain any specification paraffin from 1% to 70%. Transformation temperature of this phase-change material is adjustable from 0 to 70 deg.C. This compound phase-change material can directly apply to construction region, textile region, and martial region and so on.
Owner:HUILIN SHENGDA ENERGY MATERIAL SCI & TECH DEV BEIJING
Coated hydrous salt heat-storage material and preparation method
InactiveCN107216859AHigh phase change enthalpyImprove cycle performanceHeat-exchange elementsInorganic saltsHeat storage material
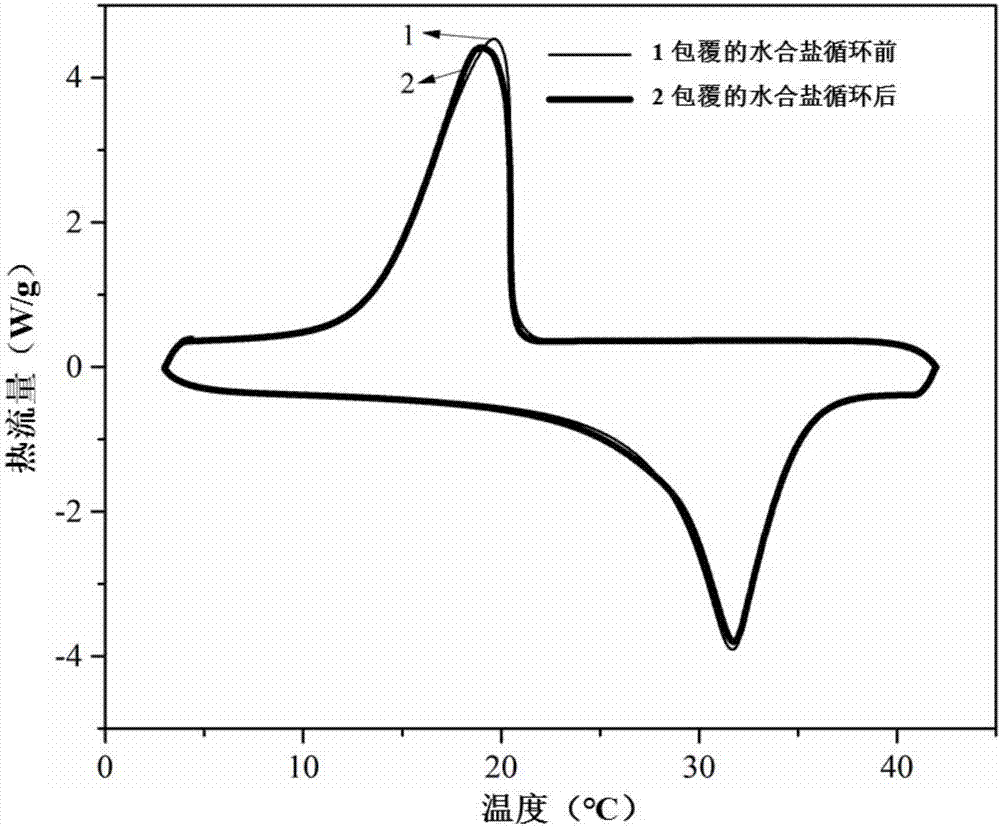
The invention discloses a coated hydrous salt heat-storage material and a preparation method. The coated hydrous salt heat-storage material is composed of an inner core material and an outer wall material. The preparation method comprises the following steps of: firstly, dissolving inorganic salt, a nucleating agent and a thickener in water to obtain a mixed solution; mixing a porous adsorption material with the mixed solution and uniformly mixing the mixture to obtain a composite material; crystallizing the composite material to prepare a compound phase change material; preparing a photocured resin solution by a resin monomer and a prepolymer; and spraying the photocured resin to the composite phase change material, and lighting the material under an UV lamp after uniform spraying to obtain the coated hydrous salt heat-storage material. The heat storage material prepared by the method has the advantages of non-phase separation, no toxicity and corrosion, small degree of supercooling, no liquid leakage and the like. Meanwhile, the heat storage material has the advantage of great heat conductivity coefficient of a carbon material, and the heat storage performance and the heat stable performance are good.
Owner:SOUTH CHINA UNIV OF TECH
Method for preparing linear polyurethane phase change material
The invention discloses a method for preparing a linear polyurethane phase change material. The method comprises the following steps: dissolving polyethylene glycol and diisocyanate into solvent in inert atmosphere, reacting in the presence of optional catalyst, adding tertiary amine type chain extender containing hydroxyl for chain extension, adding an optional neutralizer for salt formation so as to obtain the linear polyurethane phase change material, wherein the molar ratio of the sum of hydroxyl of polyethylene glycol and hydroxyl of chain extender to the isocyanate group of diisocyanate is 1:1, the add amount of the catalyst accounts for 0-1% the total weight of the polyethylene glycol, diisocyanate and chain extender, the molar ratio of neutralizer to tertiary amine type chain extender is 0-1, and the molecular weight of the polyethylene glycol is higher than 2000. The linear polyurethane phase change material prepared by the method has a linear structure and large enthalpy of phase change, has a simple preparation method, is low in cost, has stable property, can be stored for long time, is not solidified and cross-linked, is easy to process and shape, and is beneficial to large-scale popularization and application.
Owner:温州东润新材料科技有限公司
Phase change modified asphalt and preparation method thereof
InactiveCN102702759AReduces the possibility of hot ruttingControl heat diseaseBuilding insulationsToxicityLatent heat
The invention discloses phase change modified asphalt and a preparation method thereof and relates to asphalt and a preparation method of the asphalt, solving the problem of high-temperature ruts of the traditional asphalt concrete pavements. The phase change modified asphalt consists of the following components by weight: 7-8 parts of asphalt and 2-3 parts of organic phase change material. The preparation method comprises the following steps: (1) weighing the asphalt and the organic phase change material; (2) heating the weighed asphalt to 140-175 DEG C and maintaining the temperature for 3 hours; heating the weighed organic phase change material to 100 DEG C, and after the organic phase change material is completely molten into a liquid state, pouring the asphalt; and meanwhile, shearing for 20-40min by adopting a high-speed shearing and emulsifying machine to obtain the phase change modified asphalt. The phase change temperature of the phase change modified asphalt is between 40 DEG C and 75 DEG C, the phase change latent heat is larger than 25J / g, and the phase change modified asphalt has the advantages of larger phase change latent heat, no toxicity, no corrosivity and simple preparation. The invention is applied to the field of asphalt concrete pavements.
Owner:HARBIN INST OF TECH
Low-temperature calcium chloride hexahydrate heat-storage material and preparation method
InactiveCN103923613AHigh phase change enthalpyPhase transition temperature is suitableHeat-exchange elementsCelluloseCalcium Chloride Hexahydrate
The invention discloses a low-temperature calcium chloride hexahydrate heat-storage material and a preparation method. The prepared low-temperature calcium chloride hexahydrate phase-change heat-storage material comprises a nucleating agent and a thickener according to mass percent, wherein the nucleating agent is borax, alumina or sodium metasilicate nonahydrate, the thickener is sodium carboxymethl cellulose (CMC), wherein calcium chloride hexahydrate is a phase-change base material, and the usage amount of the calcium chloride hexahydrate is 95%-98%; the preparation method comprises the step of: respectively adding 1wt% of borax and 1% of CMC or 1% alumina and 4% of CMC or 1% of sodium metasilicate nonahydrate and 2% CMC for modifying the calcium chloride hexahydrate, to obtain the low-temperature calcium chloride hexahydrate heat-storage material. The phase-change latent heat of the low-temperature calcium chloride hexahydrate heat-storage material is about 150J / g, the phase-change temperature of the low-temperature calcium chloride hexahydrate heat-storage material is at 25-30DEG C, the cooling degree is less than 2DEG C, and the heat suction and release performances of the low-temperature calcium chloride hexahydrate heat-storage material after being circulated for 3000 times are stable. The low-temperature calcium chloride hexahydrate heat-storage material has excellent application prospects in agricultural facilities and residential housing.
Owner:NORTHWEST A & F UNIV
Phase-change thermoregulation textile fabric and method for preparing same
InactiveCN102704274ASimple processShort processHeating/cooling textile fabricsLiquid/gas/vapor removalBody ThermoregulationChemistry
The invention relates to an after-finishing technology of a textile fabric, in particular to a phase-change thermoregulation textile fabric and a method for preparing the phase-change thermoregulation textile fabric, belonging to the technical field of textile materials and textile material processing. According to the technical scheme disclosed by the invention, the method for preparing the phase-change thermoregulation textile fabric comprises the following steps: adding a mixture of a catalyst and citric acid as well as a cross-linking agent into polyethylene glycol solution to prepare a padding solution, wherein the catalyst is the MgCl2.6H2O, and the cross-linking agent is the dimethylol dihydroxy-ethylene urea; and subjecting polyethylene glycol and the cellulose of a cotton fabric to cross-linking reaction by adopting the polyethylene glycol cross-linking and depositing method through the padding process, the baking process and other after-finishing modes under the action of a proper amount of cross-linking agent, and depositing the product of reaction on the textile fabric to obtain the phase-change thermoregulation textile fabric. The process for preparing the phase-change thermoregulation textile fabric is simple and convenient to operate, and suitable for industrial production. The phase-change thermoregulation textile fabric prepared by adopting the method has the characteristics of washing fastness and good durability and has the bi-directional thermoregulation function, wide adaptability and obvious cost-performance ratio advantage.
Owner:JIANGSU LANSIYU HOME TEXTILE
Phase-change material for storing day/night temperature difference energy and preparation method thereof
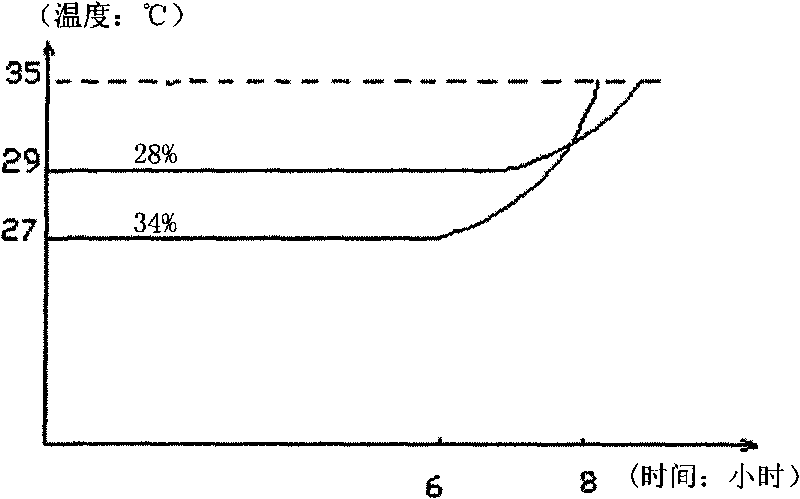
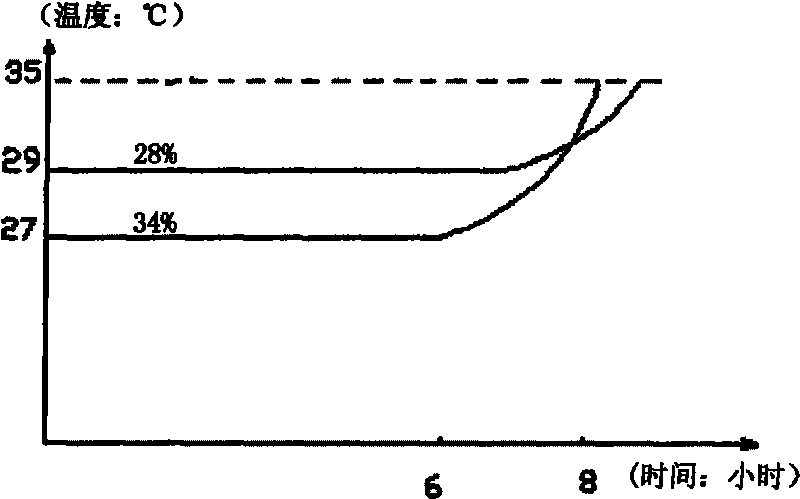
InactiveCN102079971ANot corrosiveThe phase transition process is reversibleHeat-exchange elementsWater bathsTemperature difference
The invention discloses a phase-change material applicable to storing day / night temperature difference energy and a preparation method thereof. The phase-change material is prepared by mixing CaCl2.6H2O as a storage medium with added SrCl2.6H2O and carboxymethylcellulose sodium. The preparation method comprises the steps of: respectively weighing three materials in proportion and mixing at an average valence; and heating and stirring in a constant-temperature water bath at the temperature of 40 DEG C till a mixture is completely dissolved to form a transparent liquid. In the invention, the CaCl2.6H2O has the advantages of low melting point, large heat storage capacity, safety, no toxicity and low cost and is easy to obtain. The SrCl2.6H2O is favorable for obviously reducing the subcooled temperature of the CaCl2.6H2O, and the carboxymethylcellulose sodium enables the heat storage performance of the CaCl2.6H2O to keep stable after the CaCl2.6H2O is repeatedly circulated, and can be utilized for a plurality of times. In addition, the preparation method is simple, and raw materials are low in cost and easy to obtain. The phase-change material has the phase-change temperature of 28 DEG C, and the phase-change latent heat of more than 100kJ / kg.
Owner:SHANGHAI MARITIME UNIVERSITY
Phase-change microcapsule for inhibiting pavement from being iced and preparation method
ActiveCN108300428AAvoid icingGuarantee other performanceHeat-exchange elementsMicroballoon preparationPolymer scienceMass ratio
The invention relates to a phase-change microcapsule for inhibiting a pavement from being iced and a preparation method, and belongs to the field of functional materials. The phase-change microcapsulecomprises a wall material and a core material, wherein the wall material is made from a melamine modified urea-formaldehyde resin prepolymer; the melamine modified urea-formaldehyde resin prepolymeris prepared from melamine, urea and formaldehyde according to a molar ratio of (3 to 5) to (1 to 3) to (5 to 7); the mass ratio of the wall material to the core material is (0.5 to 0.8) to 1. The phase-change microcapsule can be used for effectively controlling the low-temperature distress of an asphalt pavement, and has the advantages of being high in phase-change latent heat, simple to prepare and reversible in phase-change process, free from toxicity and corrosivity and being capable of effectively inhibiting the pavement from being iced and frosted, and the like, is used for improving theadaptive capacity, to the change of an ambient temperature, of the asphalt pavement, and is used for providing help for containing a temperature shrinkage crack and prolonging the service life of an existing pavement.
Owner:BROADVISION ENG CONSULTANTS +1
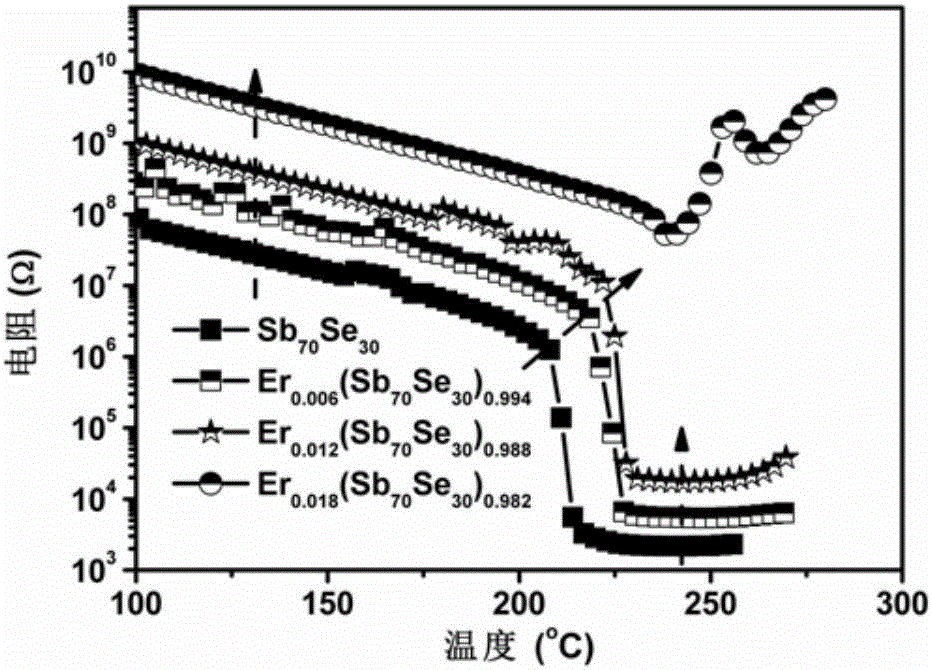
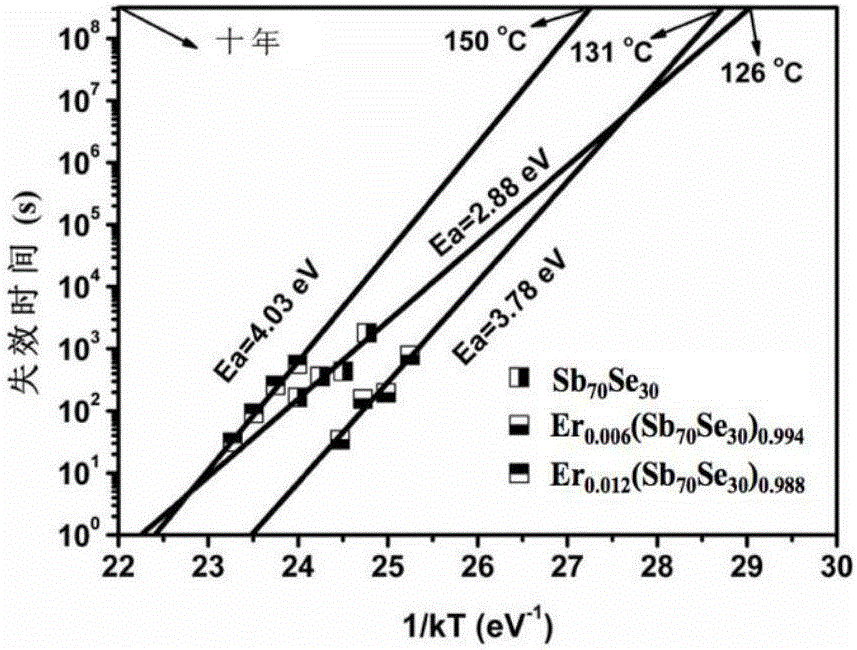
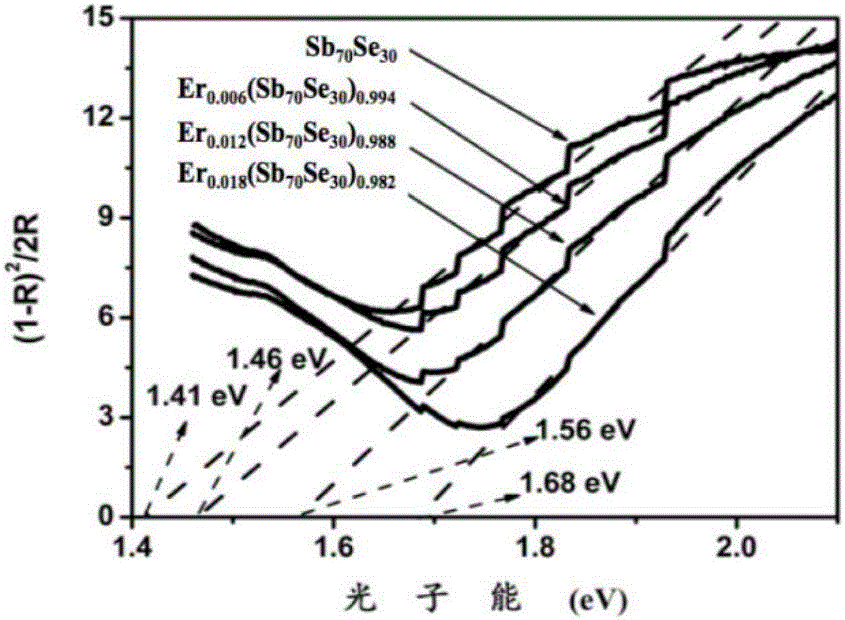
Er-Se-Sb nanometer phase change film material and preparation method and application thereof
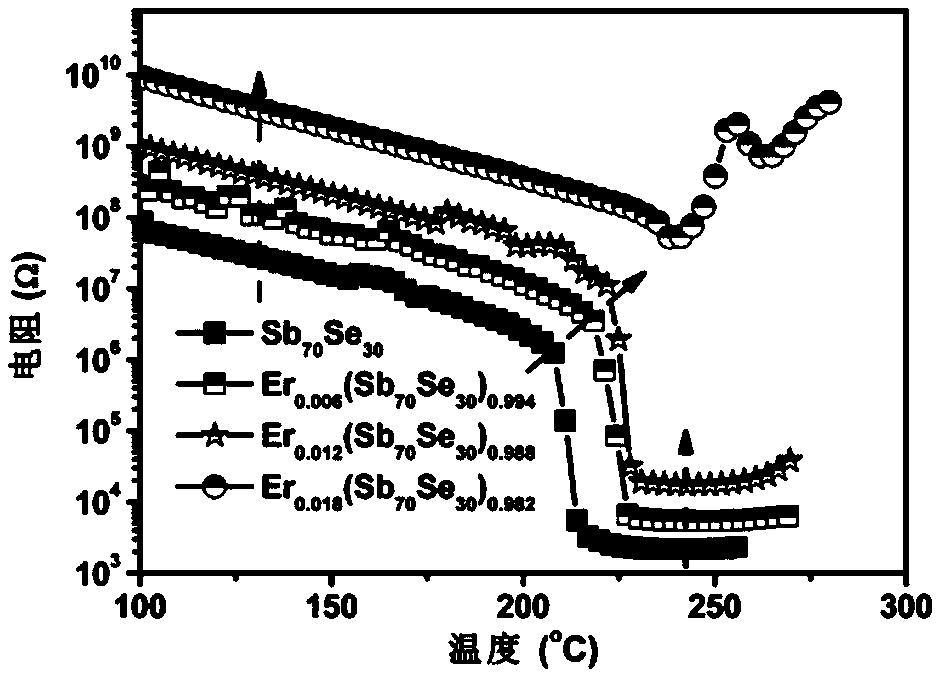
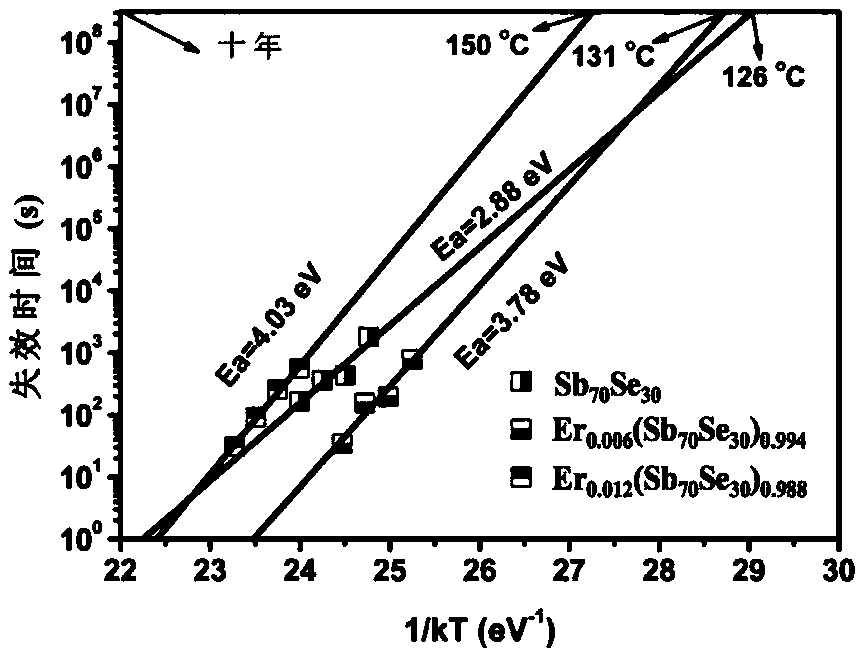
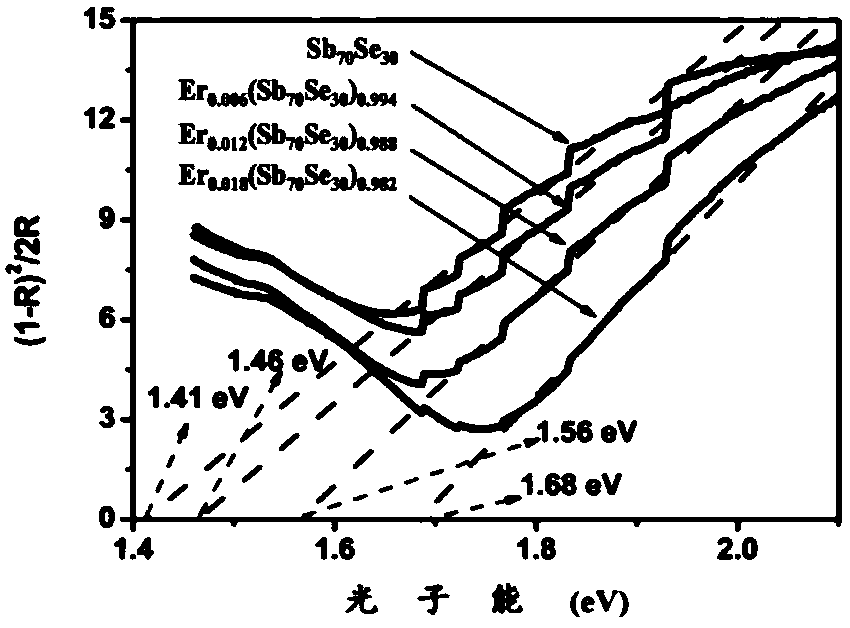
ActiveCN106229409AThe phase transition process is reversibleThe preparation method is matureMaterial nanotechnologyElectrical apparatusHigh resistanceFilm material
The invention belongs to the technical field of nanomaterials, and relates to an Er-Se-Sb nanometer phase change film material and a preparation method and an application thereof. The film material consists of the three elements of erbium, selenium and antimony, and a general chemical formula of the film material is shown as Er<x>(Se<y>Sb<100-y>)<1-x>, wherein x is greater than 0 and smaller than or equal to 0.05, and y is greater than to 0 and smaller than or equal to 50. The nanometer phase change material can realize a reversible phase change process, has a relatively large difference value between high resistance and low resistance before and after phase change, is easy for implementing '0' and '1' needing to be distinguished during storage, and is an ideal phase change storage material. The preparation method is mature, and easily compatible with an existing semiconductor technology. The nanometer phase change material inherits the advantage of high phase change speed of an antimony-rich phase, has a relatively high crystallization temperature and a data holding capability at the same time, has relatively high crystal-state and non-crystal-state resistance, and facilitates lowering of the power consumption of a phase change storage device.
Owner:JIANGSU UNIV OF TECH
Controllable temperature phase-change induction fiber and preparation method thereof
InactiveCN102677220AQuick responseFast absorbingSpinning head liquid feederConjugated cellulose/protein artificial filamentsTextile fiberPolymer science
The invention relates to a functional textile fiber material, and particularly relates to a controllable temperature phase-change induction fiber and a preparation method thereof. The preparation method comprises the steps of: mixing a regenerated silk fibroin film prepared from silk fibroin with polyethylene glycol according to the mass ratio of (9-7): (1-3), dissolving the mixture into a hexafluoroisopropanol solution, and preparing a blend spinning fluid of which the concentration is 6-20%w / w after vacuum defoaming and filtering; and controlling the distance from an injector head of a spinning container to a liquid solidification collection net at 10-15cm by adopting an electrostatic spinning process, wherein the voltage of a high-tension supply is 5-9kv, and obtaining a micro-nano fiber felt, namely the controllable temperature phase-change induction fiber. The controllable temperature phase-change induction fiber can induce absorption or discharge heat conversion function through a phase-change material when the temperature of an external environment changes, has two-way temperature phase-change induction adjustment and adaptability functions, and can be repeatedly used in a temperature oscillation environment.
Owner:SUZHOU UNIV
Lanthanide cerium-doped pure antimony pure-antimony nanometer phase-change material and preparation method thereof
InactiveCN108493337AThe preparation process is matureVery compatibleMaterial nanotechnologyElectrical apparatusPhase-change materialAntimony
The invention discloses a lanthanide cerium-doped pure antimony pure-antimony nanometer phase-change material, the component expression of which is CexSby, wherein the x and y are atomic percent, 0<x<=0.10, 0.90<y<=1, and x+y=1.00. The provided phase-change material can realize a reversible phase change process, and difference between high and low resistance before and after phase change is large,so that the material is easy to realize "0" or "1" needing to be distinguished during storage, and is a more ideal phase change storage material; and the preparation process is mature, and easy to realize compatibility with the existing semiconductor technology. The material inherits the advantages of pure antimony phase synthesis fast phase-change speed, and meanwhile, can have higher crystallization temperature and data retention, has higher crystalline-state and amorphous-state resistance, and reduces power consumption of a corresponding phase change random access memory.
Owner:JIANGSU UNIV OF TECH
Phase-change constant-temperature material
InactiveCN101760182AThe phase transition process is reversibleLow priceHeat-exchange elementsCelluloseMass ratio
The invention discloses a phase-change constant-temperature material which contains the following components in the mass ratio: 28-34 percent of CaCl2.6H2O, 40-45 percent of NaSO4.10H2O, 5-8 percent of Na2B4O7.10H2O, 10-27 percent of water and 8 percent of cellulose. The phase-change temperature of the phase-change material provided by the invention is 26-29 DEG C, the latent heat of phase change is 198-257KJ / KG, the phase-change process is reversible, the recyclable use frequency is more than 2000 times, all the raw materials are produced in a large scale, and the phase-change material has low price, no toxicity, no corrosion and easy popularization. The phase-change material is in a white snow floc shape at normal temperature, has a certain elasticity, is very easy for plasticity and is convenient for development and utilization in various energy-storage fields.
Owner:肖吕明
Carbon fiber-added microcapsule shape-stabilized phase-change material and preparation method thereof
InactiveCN106753262AHigh mechanical strengthNo brokenHeat-exchange elementsMicroballoon preparationFiberPolymerization
The invention discloses a carbon fiber-added microcapsule shape-stabilized phase-change material and a preparation method thereof. The carbon fiber-added microcapsule shape-stabilized phase-change material comprises the following components in parts by weight: 50 to 100 parts of a core material, 200 to 500 parts of chloroform, 0.2 to 0.6 part of an emulsifying agent, 15 to 30 parts of toluene diisocynate, 6 to 15 parts of ethidene diamine and 0 to 100 parts of distilled water, wherein the core material comprises paraffin, polyethylene and carbon fiber powder, and the weight ratio of the paraffin to the polyethylene to the carbon fiber powder is (30-70):(20-50):(5-15). The microcapsule shape-stabilized phase-change material provided by the invention adopts the polyethylene and the carbon fiber as composite supporting materials, the mechanical strength of the microcapsules is greatly enhanced, the microcapsules are not broken when 10-Kg pressure is applied, and the microcapsule shape-stabilized phase-change material can be widely applied to various aspects in the field of buildings and in the fields of military, civil use, household appliances and the like. The carbon fiber-added microcapsule shape-stabilized phase-change material is wrapped by an interface polymerization method, loss of the paraffin can be retarded or stopped, heat storage and release functions of the phase-change material are guaranteed, the service life of the phase-change material is prolonged (after heat storage and release is conducted for 50 times, attenuation is zero), and the carbon fiber-added microcapsule shape-stabilized phase-change material can adapt to more use environments.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Phase-change material with phase-change temperature of minus 15 DEG C
ActiveCN103254876ALarge latent heat of phase changeImprove performanceChemical industryHeat-exchange elementsCarboxymethyl celluloseAlum
The invention discloses a phase-change material with the phase-change temperature of minus 15 DEG C. The phase-change material comprises the following components in percentage by weight: 5-25 percent of ammonium chloride, 2.5-25 percent of crylic acid, 5-15 percent of alum, 5-25 percent of maleic anhydride, 5-10 percent of carboxymethyl cellulose sodium, 5-25 percent of oil and the balance of water. Due to the mode, the phase-change material with the phase-change temperature of minus 15 DEG C is large in latent heat of phase change, stable in performance, reversible in phase change process, wide in raw material source, low in cost, safe and reliable, has no phase separation phenomenon after being used for a long time and can be circularly used for more than ten thousand times; the consumption of electric energy is reduced; the positive effects of saving energy and reducing emission are achieved; and the phase-change material can be popularized and applied in refrigerating and freezing processes in food stuff, medicines and the like.
Owner:SUZHOU ANTEK INDAL
Phase change energy storage material prepared from oleic acid or esters of energy storage material and preparation method thereof
ActiveCN102010692AWide variety of sourcesLow priceOrganic compound preparationCarboxylic acid esters preparationPhase changeLatent heat
A phase change energy storage material prepared from oleic acid or esters of the energy storage material is characterized in that the general molecular formula of the material is CH3(CH2)7CHOH-CHOH(CH2)7COOR, wherein R is H, CH3 or CH2CH3. The material belongs to the compound of fatty acid derivatives and has phase change temperature between 35 DEG C and 130 DEG C and phase change latent heat of 110-206kJ / kg. The material has the advantages of controllable phase change temperature and wider range of phase change temperature.
Owner:SHANXI INST OF COAL CHEM CHINESE ACAD OF SCI
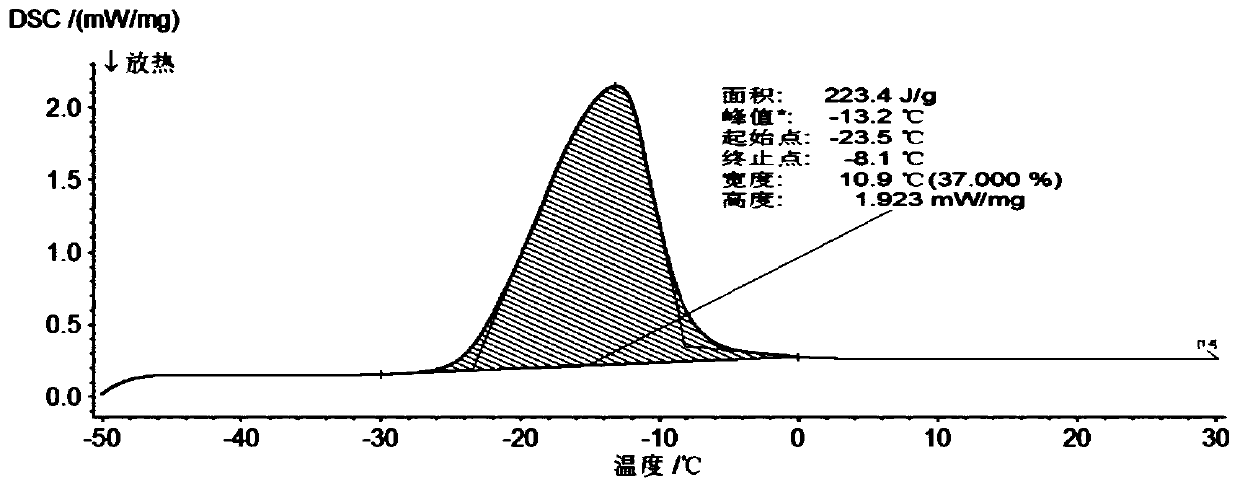
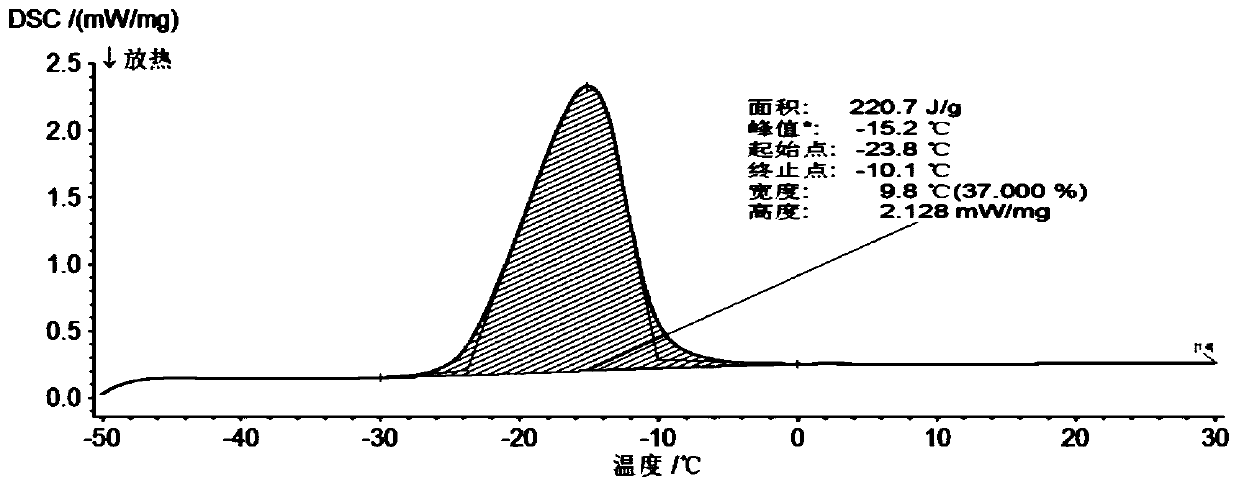
A jelly-like gel-type composite low-temperature phase-change cold storage material and its preparation method
The invention provides a jelly-like gel type composite low-temperature phase-change cold storage material. The jelly-like gel type composite low-temperature phase-change cold storage material is prepared from the following components in percentage by mass: 22 to 23 percent of sodium formate, 12 percent of potassium chloride, 0.4 percent of xanthan gum, 0.12 percent of nanometer titanium dioxide, 0.13 percent of lauryl sodium sulfate and the balance of distilled water. The invention also provides a preparation method of the low-temperature phase-change cold storage material. The phase change temperature of the material is -23.5 to -23.8 DEG C, and the latent heat is 220.7 to 235.7J / g. The jelly-like gel type composite low-temperature phase-change cold storage material is reversible in a phase change process, is in the form of jelly-like gel at room temperature, and can be prevented from leaking; required raw materials are stable in chemical properties, can be used for a long time, and are non-toxic, harmless, wide in sources and lower in cost, so that the cold storage material can be widely applied to the low temperature field.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
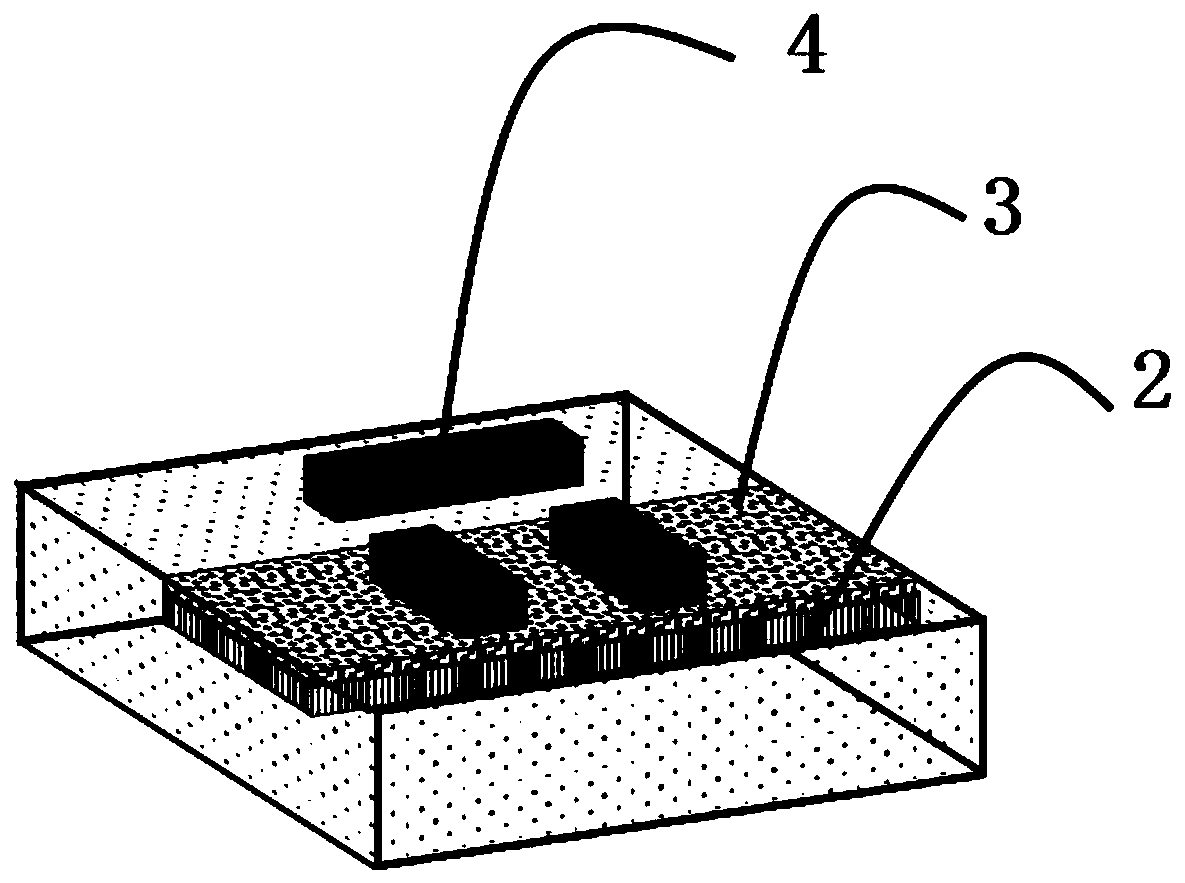
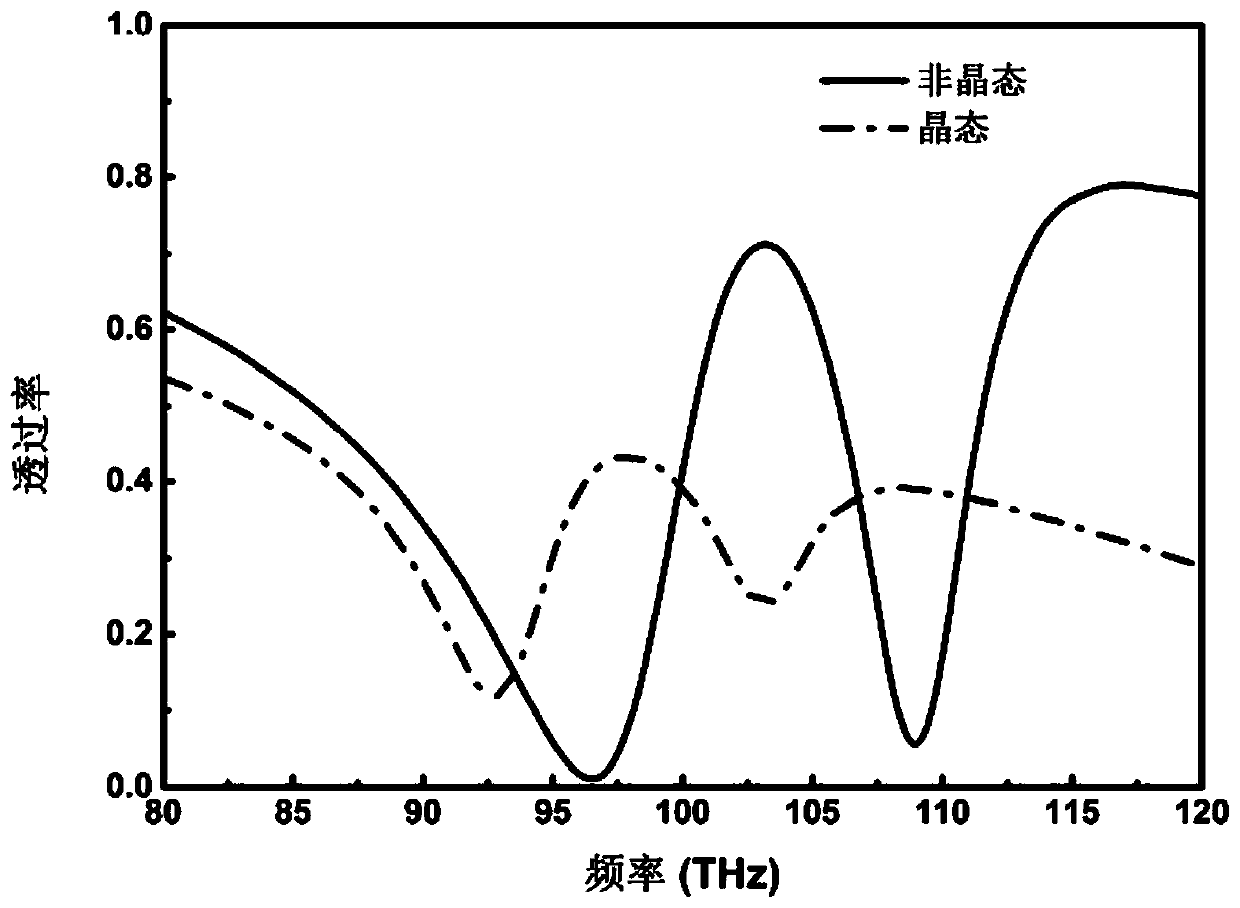
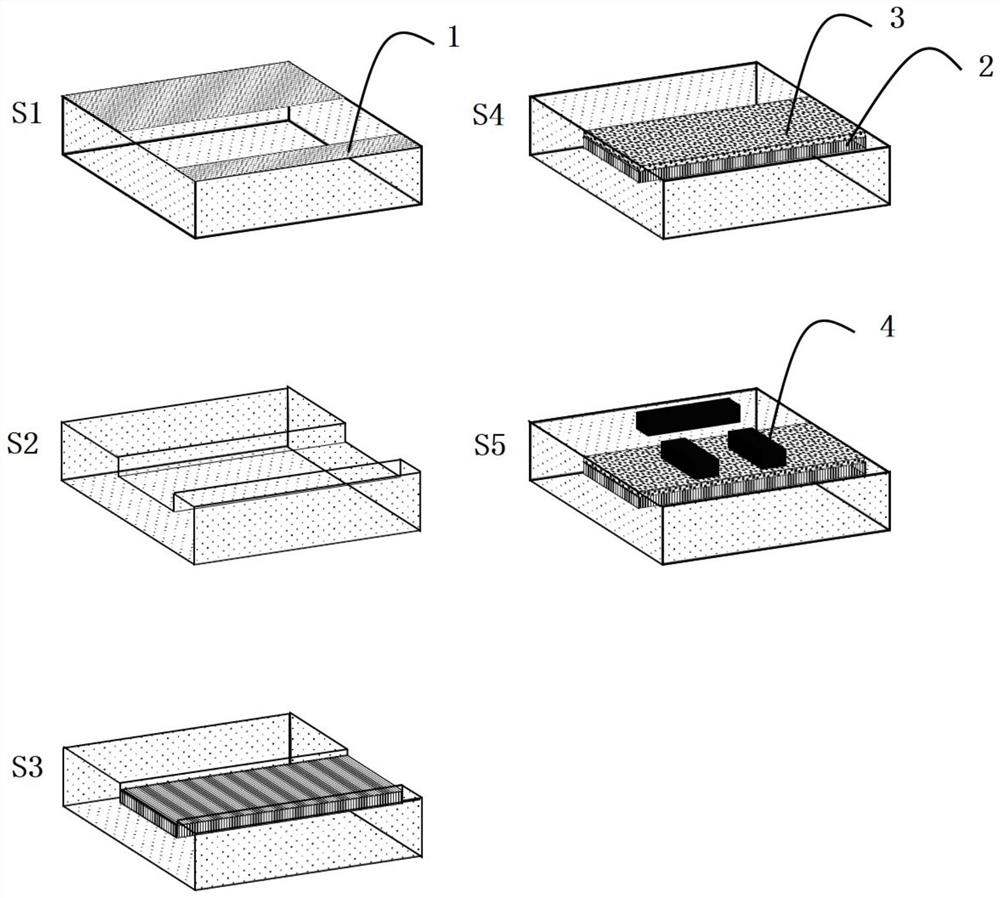
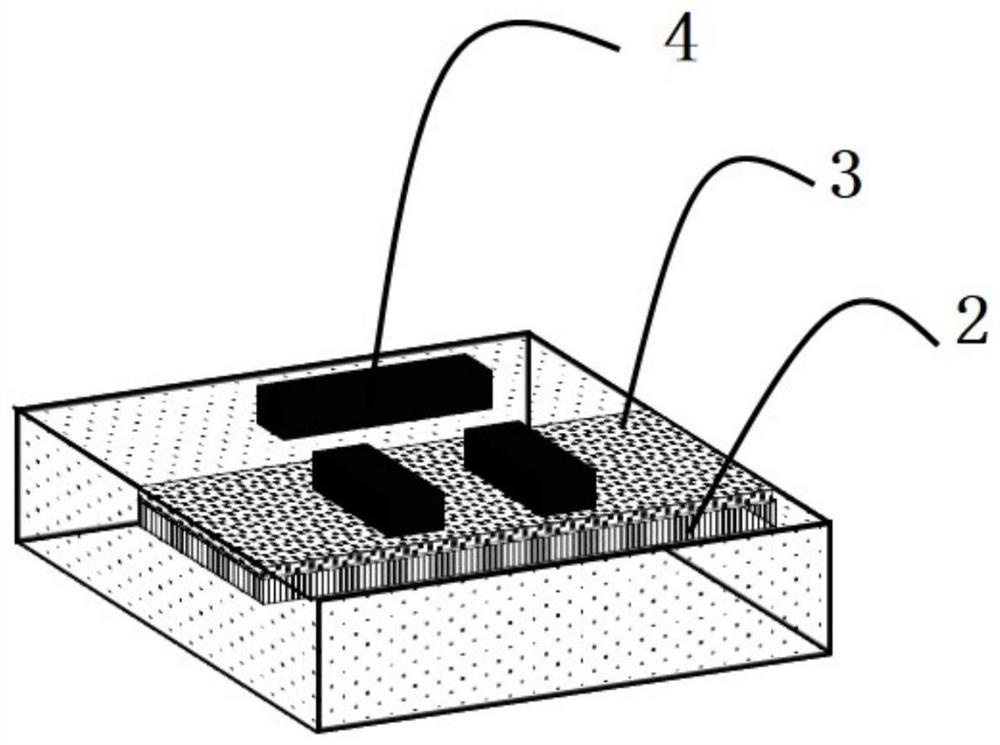
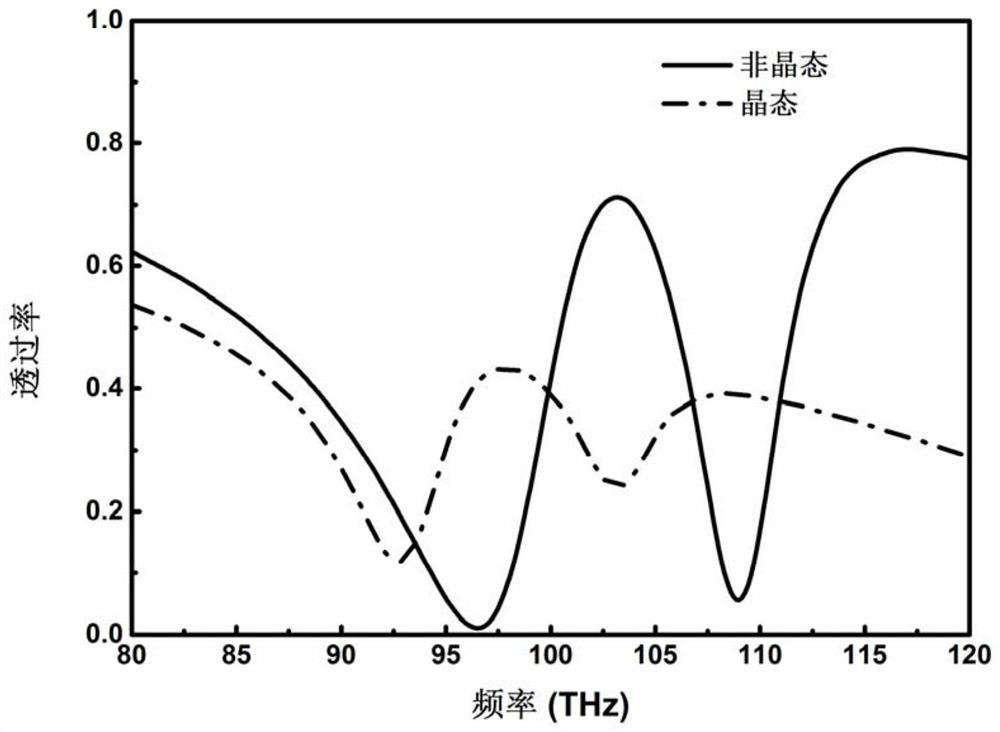
Phase-changing material-based adjustable slow light device and fabrication method and application thereof
ActiveCN110764283AWide frequency rangeIncrease the amount of changeNon-linear opticsSlow lightDielectric substrate
The invention discloses a phase-changing material-based adjustable slow light device. The adjustable slow light device comprises a dielectric substrate, a phase-changing material, a dielectric protection layer and a metal structure which are sequentially arranged. The invention also provides fabrication method and application of the phase-changing material-based adjustable slow light device. The phase-changing material and a metal periodic pattern structure are combined, and control of slow light of the device is achieved by various stimulation means such as light, power and by means of various advantages of wide dielectric constant change frequency range, large change quantity and versatile phase-changing conditions of the phase-changing material. Moreover, the device also has the characteristics of wide integration, wide working frequency range and processing flexibility.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
Phase-change constant-temperature material
InactiveCN101760182BThe phase transition process is reversibleLow priceHeat-exchange elementsCellulosePhase change
The invention discloses a phase-change constant-temperature material which contains the following components in the mass ratio: 28-34 percent of CaCl2.6H2O, 40-45 percent of NaSO4.10H2O, 5 or 8 percent of Na2B4O7.10H2O, 10-27 percent of water and 8 percent of cellulose. The phase-change temperature of the phase-change material provided by the invention is 26-29 DEG C, the latent heat of phase change is 198-257KJ / KG, the phase-change process is reversible, the recyclable use frequency is more than 2000 times, all the raw materials are produced in a large scale, and the phase-change material has low price, no toxicity, no corrosion and easy popularization. The phase-change material is in a white snow floc shape at normal temperature, has a certain elasticity, is very easy for plasticity and is convenient for development and utilization in various energy-storage fields.
Owner:肖吕明
Organic mix phase-changing material for air conditioner cold accumulation
InactiveCN101220259ANo phase separationThe phase transition process is reversibleHeat-exchange elementsCoefficient of performanceLiquidus
The invention relates to an organic additive phase-change material used for air conditioning cold storage, and is prepared by forming an organic eutectic body of aminoethanol with mass ratio of 99.4 percent-98 percent and 0.6-2 percent of water, wherein, aluminium powder with a mass ratio of 1-5 percent, and particle size of 1-4Mum is added and mixed into the eutectic body. The phase-change temperature of the material is 6-9.2 DEG C; the phase-change latent heat is 155-196kJ / kg; the liquidus thermal conductivity is 0.27-0.48W / (m.k). The invention has the advantages that the phase-change temperature is proper; the latent heat is high; the condenser depression is low; the chemical stability is good; the thermal conductivity is high; in the application of the invention to the air conditioning cold storage system, a general refrigerating battery can be directly adopted for the cold storage, thus effectively improving the property coefficient of the refrigerating battery and lowering the energy consumption of the system.
Owner:贾代勇 +3
Inorganic phase-change constant-temperature material and preparation method thereof
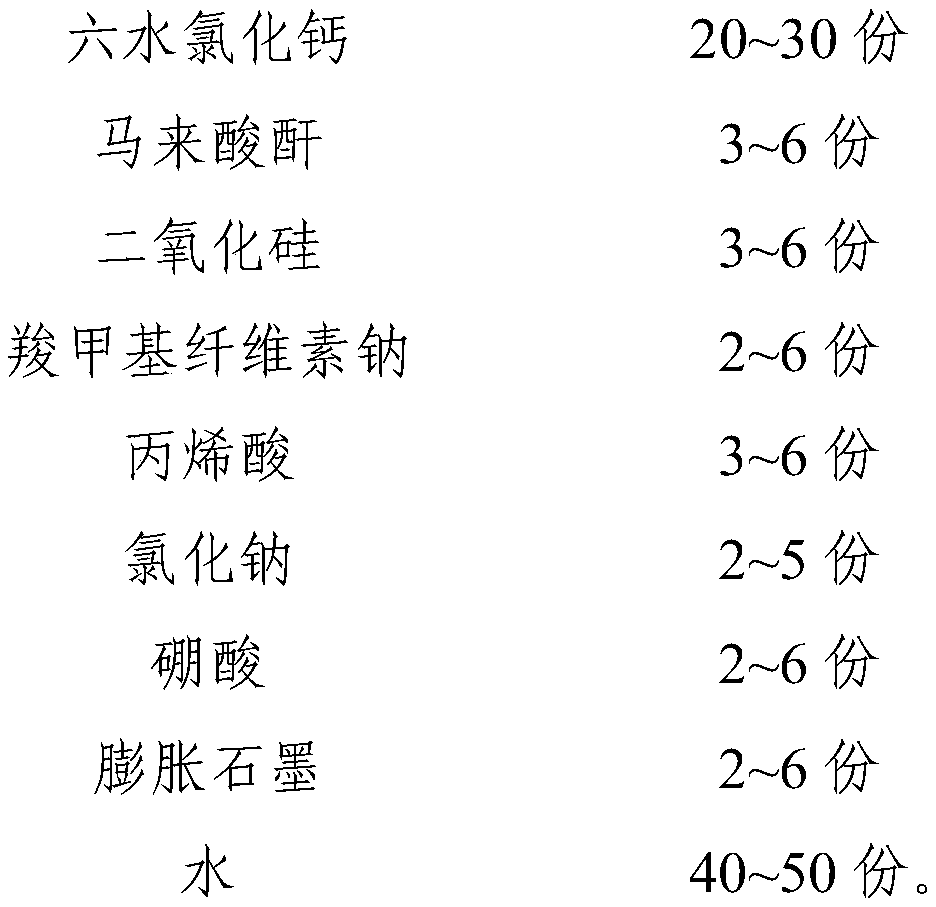
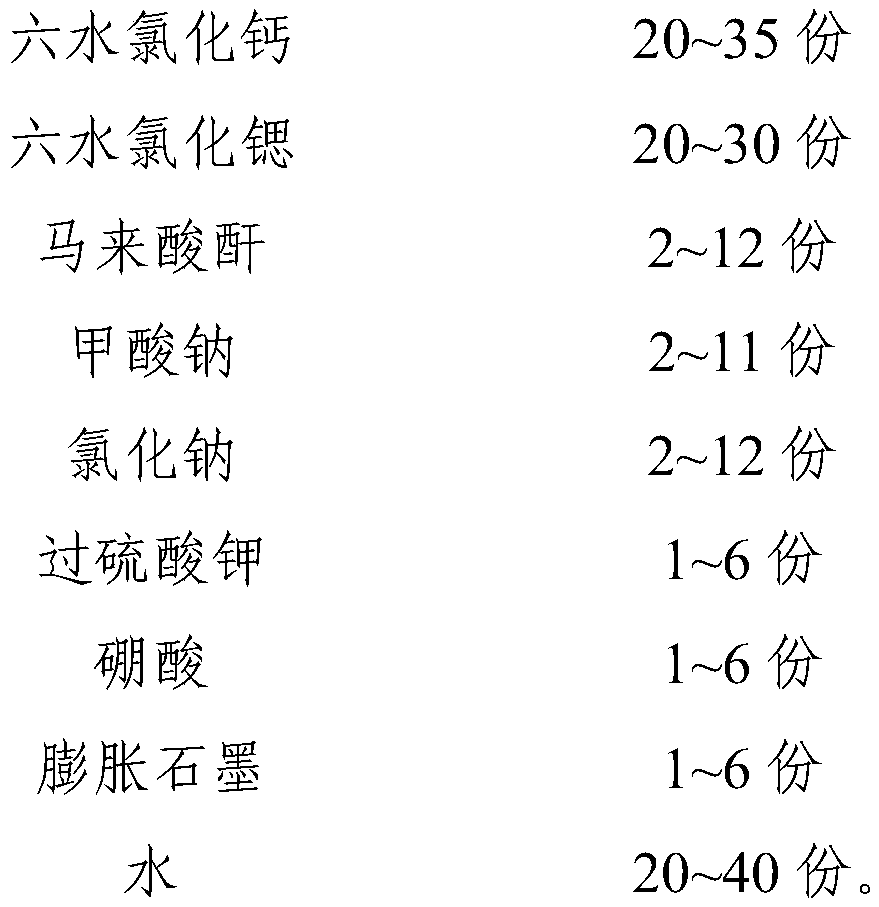
InactiveCN111087977APrevent leakageImprove performanceHeat-exchange elementsCelluloseCalcium Chloride Hexahydrate
The invention relates to a phase-change material, specifically to an inorganic phase-change constant-temperature material and a preparation method thereof. The inorganic phase-change constant-temperature material comprises calcium chloride hexahydrate, maleic anhydride, silicon dioxide, sodium carboxymethyl cellulose, acrylic acid, sodium chloride, boric acid and expanded graphite, wherein the total amount of the boric acid and the expanded graphite accounts for 4-12% of the total amount of the inorganic phase-change material; and the mass ratio of the boric acid to the expanded graphite is (2-6): (2-6). The inorganic phase-change constant-temperature material provided by the invention has a phase-change temperature of 17 DEG C, a supercooling degree of 0.3 DEG C and a phase-change latentheat of no lower than 189 KJ / Kg; the phase-change process is reversible, and the number of times of recycling is not lower than 10000; in the circulation process, the situation that the thermophysicalproperty is degraded does not exist; meanwhile, the inorganic phase-change constant-temperature material is not prone to leaking from a base body.
Owner:北京中海前沿材料技术有限公司
A kind of method for preparing linear polyurethane phase change energy storage material
The invention discloses a method for preparing a linear polyurethane phase change material. The method comprises the following steps: dissolving polyethylene glycol and diisocyanate into solvent in inert atmosphere, reacting in the presence of optional catalyst, adding tertiary amine type chain extender containing hydroxyl for chain extension, adding an optional neutralizer for salt formation so as to obtain the linear polyurethane phase change material, wherein the molar ratio of the sum of hydroxyl of polyethylene glycol and hydroxyl of chain extender to the isocyanate group of diisocyanate is 1:1, the add amount of the catalyst accounts for 0-1% the total weight of the polyethylene glycol, diisocyanate and chain extender, the molar ratio of neutralizer to tertiary amine type chain extender is 0-1, and the molecular weight of the polyethylene glycol is higher than 2000. The linear polyurethane phase change material prepared by the method has a linear structure and large enthalpy of phase change, has a simple preparation method, is low in cost, has stable property, can be stored for long time, is not solidified and cross-linked, is easy to process and shape, and is beneficial to large-scale popularization and application.
Owner:温州东润新材料科技有限公司
Tunable slow light device based on phase change material, its preparation method and application
ActiveCN110764283BAdjustable dielectric constantThe phase transition process is reversibleNon-linear opticsSlow lightDielectric substrate
The invention discloses a phase-changing material-based adjustable slow light device. The adjustable slow light device comprises a dielectric substrate, a phase-changing material, a dielectric protection layer and a metal structure which are sequentially arranged. The invention also provides fabrication method and application of the phase-changing material-based adjustable slow light device. The phase-changing material and a metal periodic pattern structure are combined, and control of slow light of the device is achieved by various stimulation means such as light, power and by means of various advantages of wide dielectric constant change frequency range, large change quantity and versatile phase-changing conditions of the phase-changing material. Moreover, the device also has the characteristics of wide integration, wide working frequency range and processing flexibility.
Owner:INST OF PHYSICS - CHINESE ACAD OF SCI
A kind of er-se-sb nano phase change film material and its preparation method and application
ActiveCN106229409BThe phase transition process is reversibleThe preparation method is matureMaterial nanotechnologyElectrical apparatusHigh resistanceFilm material
The invention belongs to the technical field of nanomaterials, and relates to an Er-Se-Sb nano-phase change thin film material and a preparation method and application thereof. The thin film material of the present invention is composed of three elements, erbium, selenium and antimony, and its general chemical formula is Erx(SeySb100-y)1-x, wherein 0
Owner:JIANGSU UNIV OF TECH
A kind of phase change microcapsule for suppressing road icing and preparation method thereof
ActiveCN108300428BAvoid icingGuarantee other performanceHeat-exchange elementsMicroballoon preparationRoad surfaceMelamine
The invention relates to a phase-change microcapsule for inhibiting a pavement from being iced and a preparation method, and belongs to the field of functional materials. The phase-change microcapsulecomprises a wall material and a core material, wherein the wall material is made from a melamine modified urea-formaldehyde resin prepolymer; the melamine modified urea-formaldehyde resin prepolymeris prepared from melamine, urea and formaldehyde according to a molar ratio of (3 to 5) to (1 to 3) to (5 to 7); the mass ratio of the wall material to the core material is (0.5 to 0.8) to 1. The phase-change microcapsule can be used for effectively controlling the low-temperature distress of an asphalt pavement, and has the advantages of being high in phase-change latent heat, simple to prepare and reversible in phase-change process, free from toxicity and corrosivity and being capable of effectively inhibiting the pavement from being iced and frosted, and the like, is used for improving theadaptive capacity, to the change of an ambient temperature, of the asphalt pavement, and is used for providing help for containing a temperature shrinkage crack and prolonging the service life of an existing pavement.
Owner:BROADVISION ENG CONSULTANTS +1
A kind of phase change heat storage material and its preparation method and application
ActiveCN103666378BThe phase transition process is reversibleReduce subcoolingHeat-exchange elementsHeat storage materialSodium Acetate Trihydrate
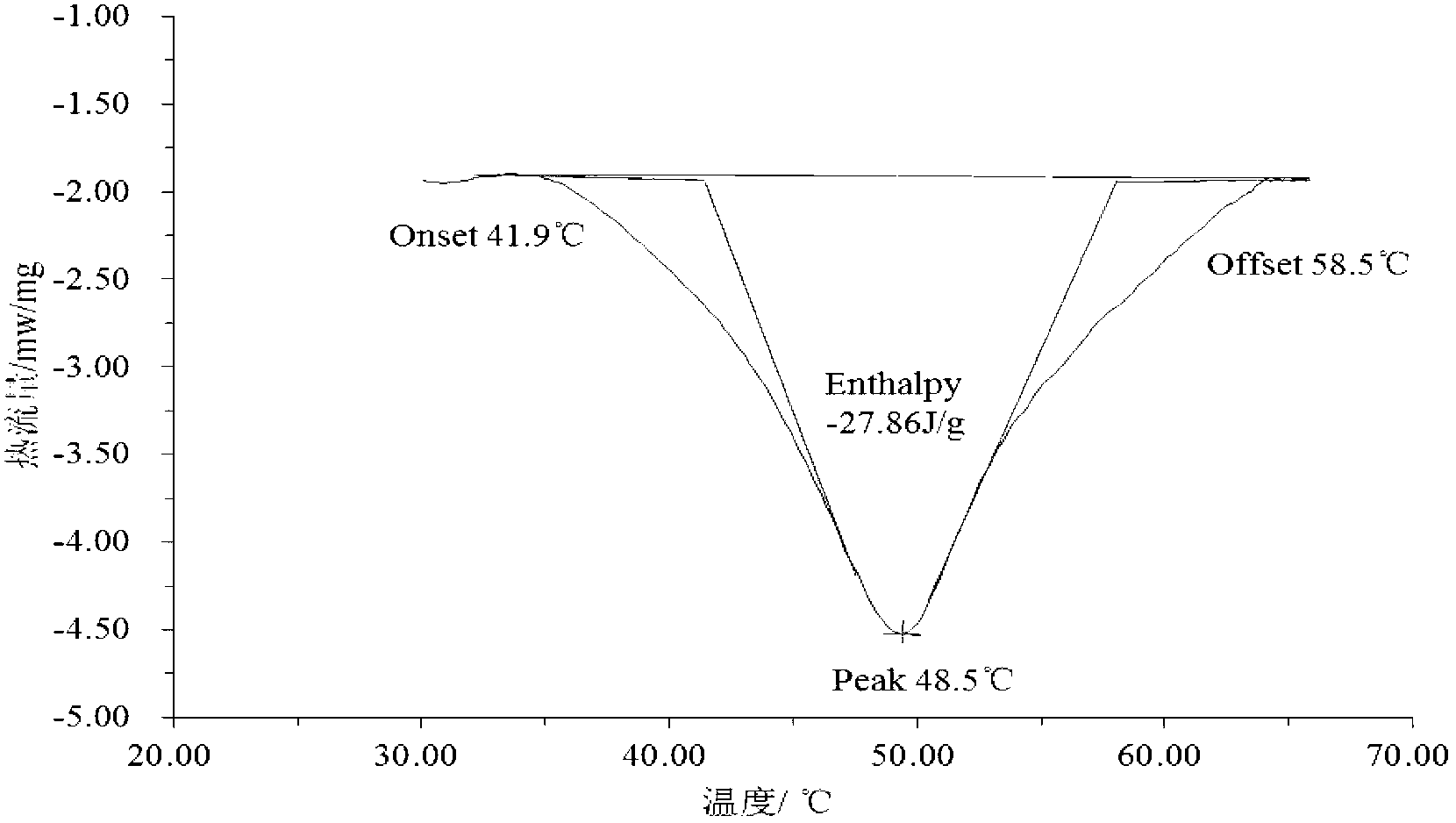
The invention discloses a phase change heat storage material and a preparation method thereof. The phase change heat storage material comprises the following raw materials in parts by weight: 55-70 parts of sodium acetate trihydrate, 1-5 parts of a phase change point conditioning agent, 20-30 parts of a softening agent, 1.08 parts of a nucleating agent, 0.5-1 part of an overcooling-proof agent and 5-15 parts of water. The phase change temperature of the prepared phase change heat storage material is 50-55 DEG C, the latent heat of phase change is 200-250 KJ / kg, the phase change process is reversible, the degree of overcooling is low, softness can be kept after phase change crystallization, and the application potentiality is high in various heat storage fields. The phase change heat storage material is soft and elastic, and can be used for heat storage type heating pads which are particularly used after shower; the phase change heat storage material can also be used for indoor heating appliances of foot pads, cushions and the like.
Owner:ZHEJIANG TRIPLE WIN MEDICAL APPLIANCE
Inorganic phase-change constant-temperature material and preparation method thereof
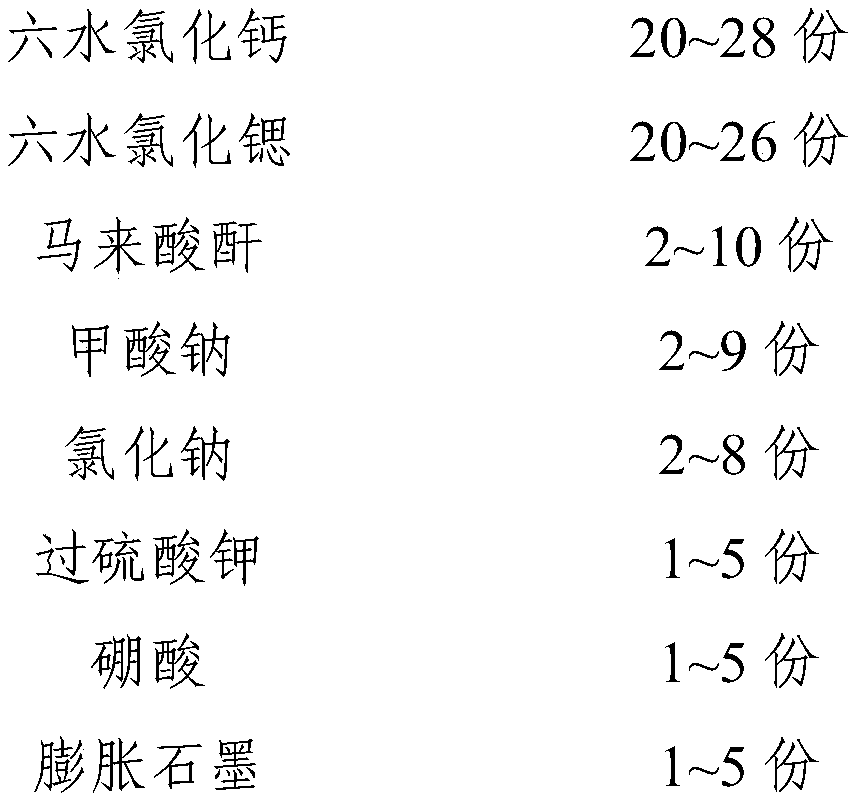
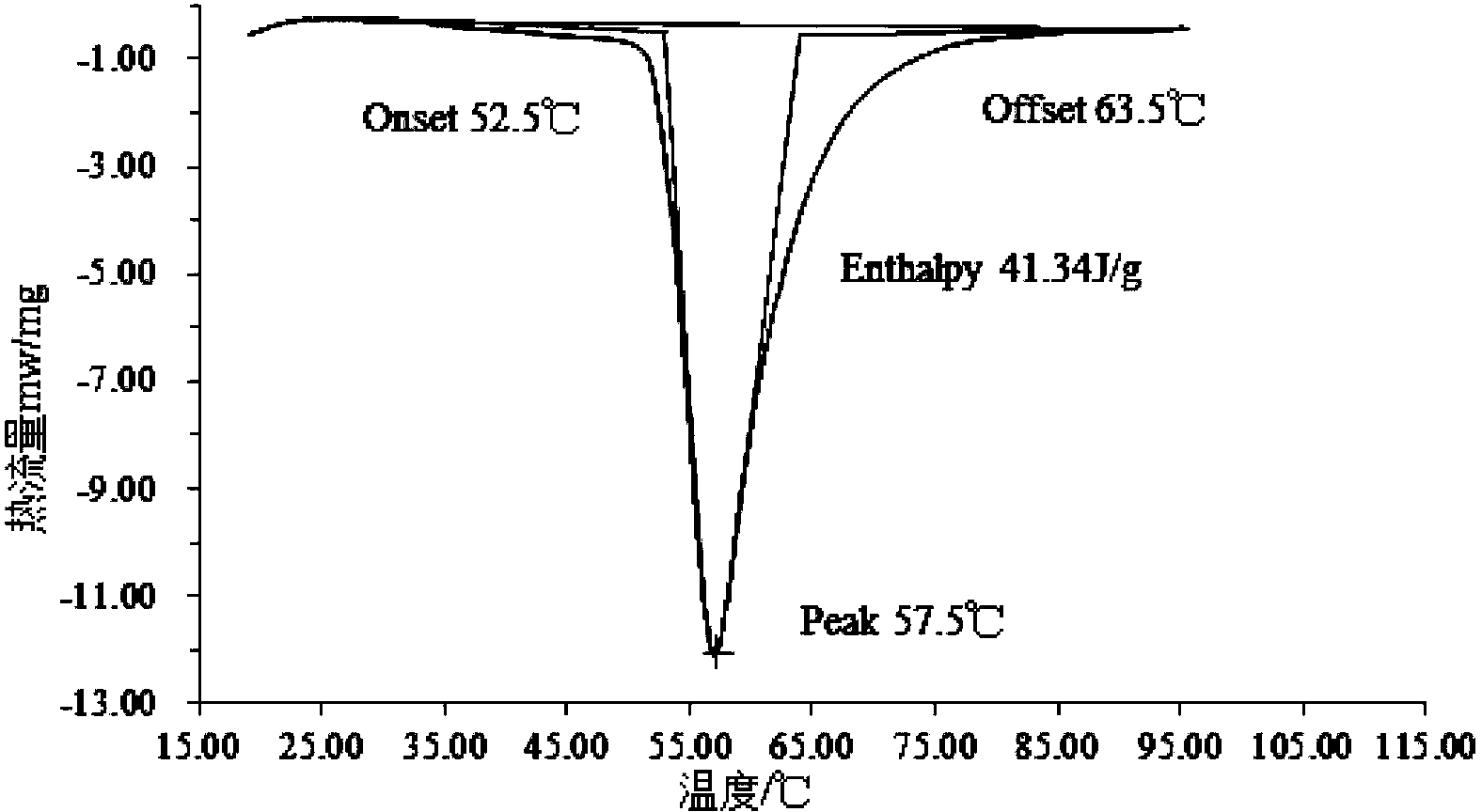
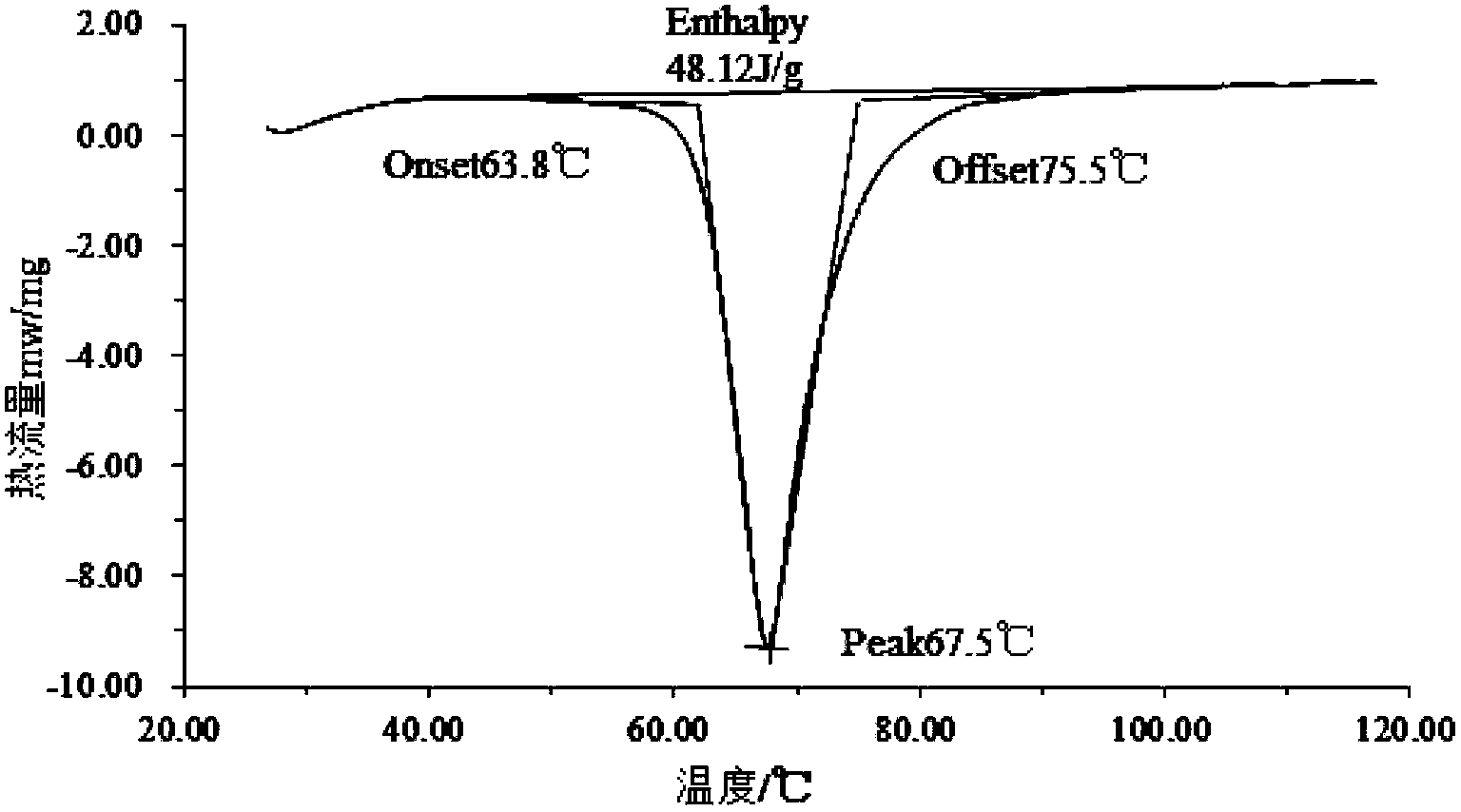
InactiveCN111073605APrevent leakageImprove performanceHeat-exchange elementsStrontium chloride hexahydratePotassium persulfate
The invention relates to a phase-change material, in particular to an inorganic phase-change constant-temperature material and a preparation method thereof. The inorganic phase-change constant-temperature material comprises calcium chloride hexahydrate, strontium chloride hexahydrate, maleic anhydride, sodium formate, sodium chloride, potassium persulfate, boric acid and expanded graphite; whereinthe total amount of the boric acid and the expanded graphite accounts for 2-12% of the total amount of the inorganic phase-change constant-temperature material; wherein the mass ratio of the boric acid to the expanded graphite is (1-6): (1-6). According to the inorganic phase-change constant-temperature material, the phase-change temperature is 39 DEG C, the supercooling degree is 0.3 DEG C, thephase-change latent heat is not lower than 221 KJ / Kg, the phase-change process is reversible, and the number of times of recycling is not lower than 10000; and in addition, the situation of thermal physical property degradation does not exist in the circulation process, and leakage from a base body is not likely to happen.
Owner:北京中海前沿材料技术有限公司
Ceramic sand-like composite phase change material and preparation method thereof
The invention provides a ceramic sand-like composite phase change material and a preparation method thereof, and relates to a phase change material and a preparation method thereof. The purpose of the present invention is to solve the problem of high temperature rutting on the existing asphalt concrete pavement. The material of the present invention comprises, by weigh, 7-8 parts of ceramic sand, and 2-3 parts of an organic phase change material. The preparation method comprises the following step: 1, weighing the ceramic sand and the organic phase change material; 2, carrying out crushing grinding, screening, water washing, and drying on the ceramic sand; 3, uniformly mixing the organic phase change material and the ceramic sand according to a mass ratio of 3:7, carrying out vacuum drying, adding mineral powder with a volume fraction of 0.5%, and carrying out drying and screening to obtain a composite material; 4, immersing the composite material in an epoxy emulsion, and holding for 3-5 s, and spreading iron powder on the surface to obtain a mixed material; and 5, repeated performing the step 4 3-5 times to obtain the material of the present invention. The material of the present invention has a phase change temperature of 40-75 DEG C and a phase change latent heat more than 40 kJ / kg, and is applicable for the field of asphalt concrete pavements.
Owner:HARBIN INST OF TECH
A phase change material with a phase change temperature of -20°C
The invention discloses a phase-change material with a phase-change temperature of -20 DEG C. The phase-change material comprises, by weight, 5 to 25% of ammonium chloride, 3.5 to 25% of acrylic acid, 5 to 15% of alum, 5 to 25% of maleic anhydride, 5 to 10% of carboxymethyl cellulose sodium and 10 to 25% of grease, with the balance being water. According to the invention, the phase-change material with the phase-change temperature of -20 DEG C has the advantages of widely available raw materials, low cost, no toxic and side effects, safety, reliability, great latent heat of phase change, stable performances, no phase separation, reversible phase change process, capacity of being reused more than ten thousands of times, reduction of electric energy consumed in the processes of transport and storage and active effects of energy saving and emission reduction.
Owner:SUZHOU ANTEK INDAL
Low-temperature phase change cold storage material
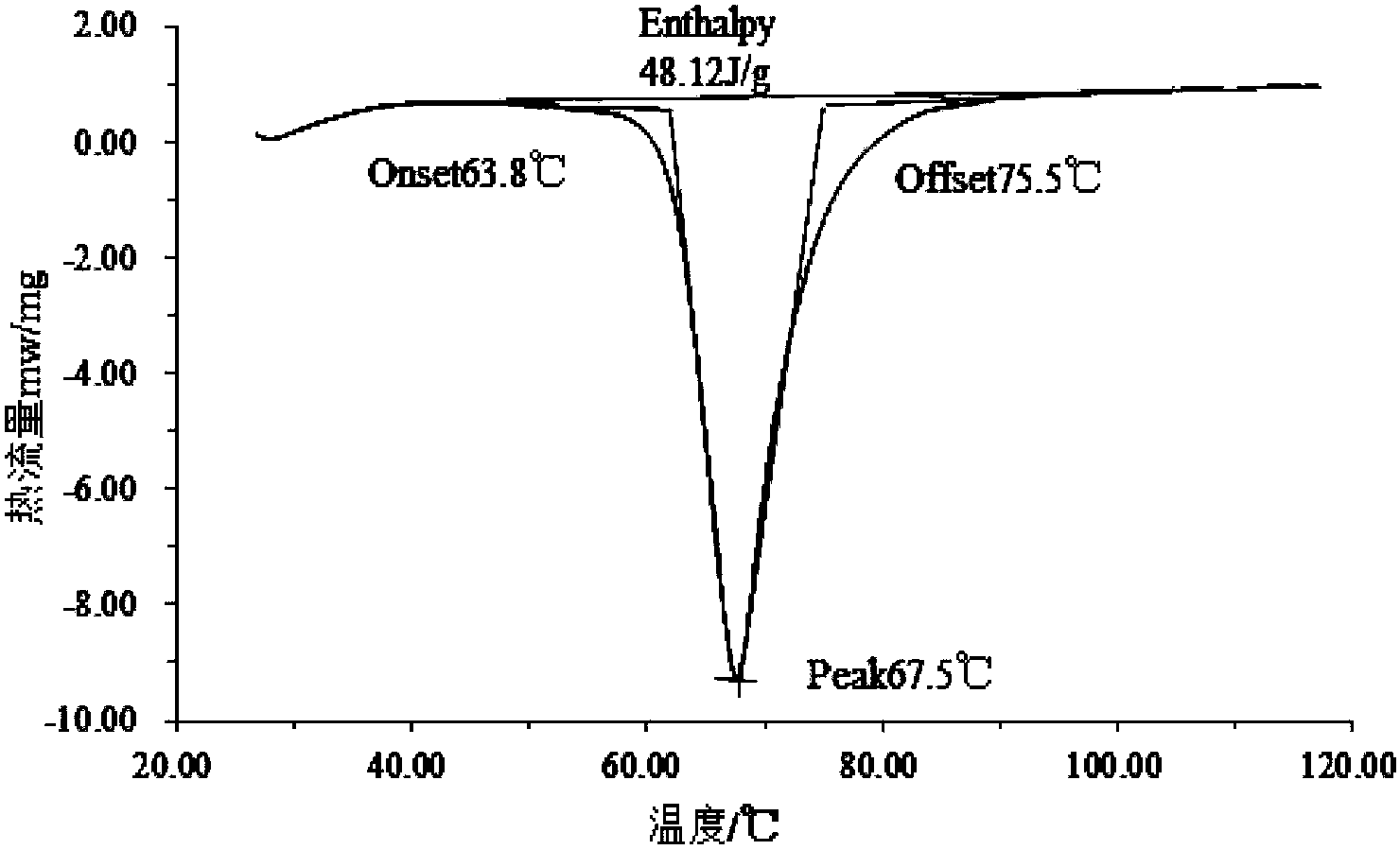
PendingCN114181672AThe phase transition process is reversibleHeat-exchange elementsPhase change enthalpyGlycerol
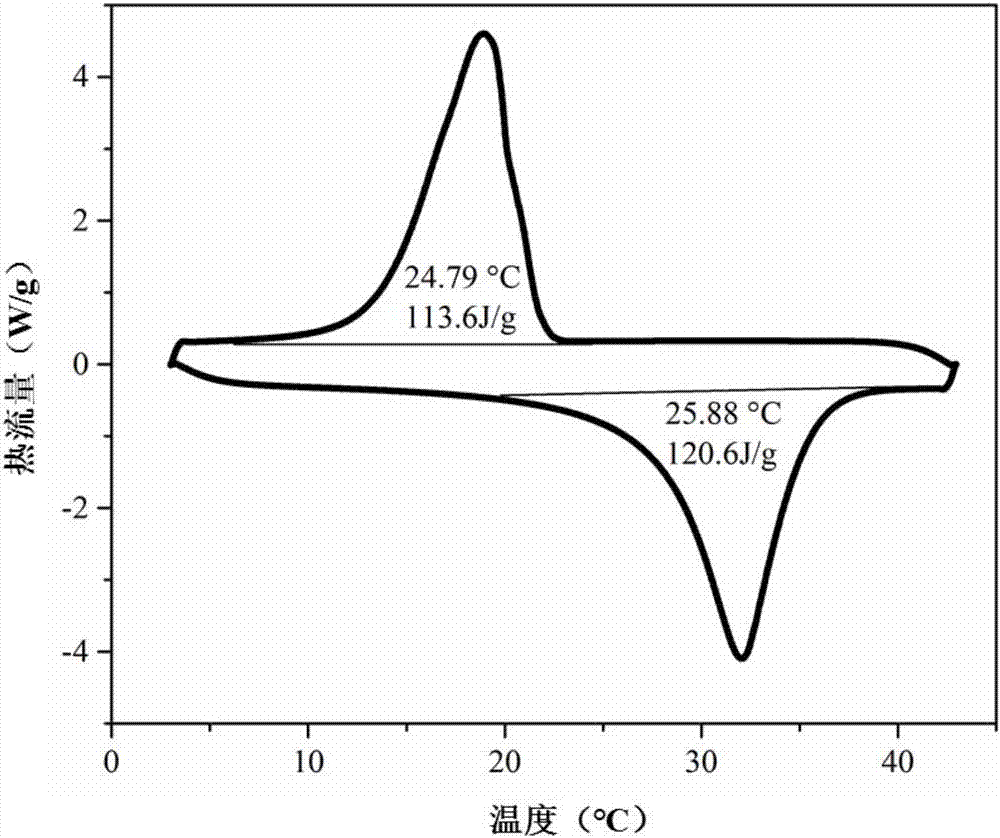
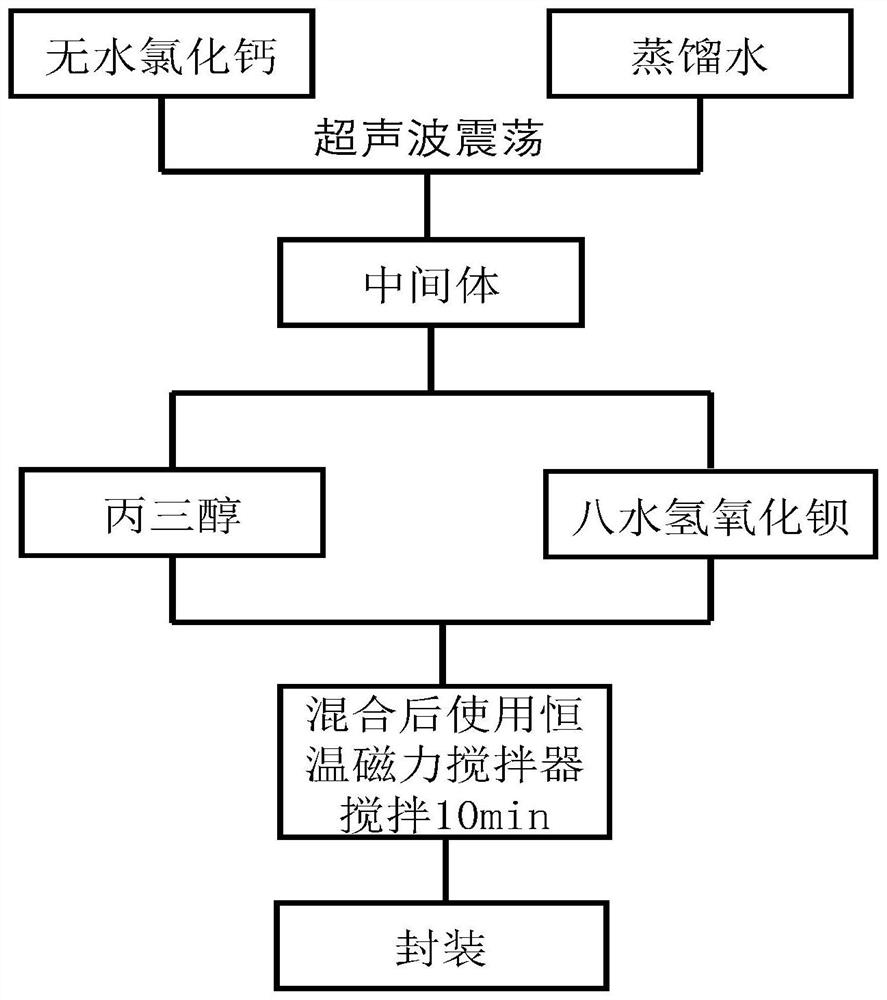
The invention discloses a low-temperature phase-change cold storage material, and relates to an organic-inorganic composite phase-change material, which comprises anhydrous calcium chloride, distilled water, glycerol and barium hydroxide octahydrate, and the composite phase-change material contains the following components in percentage by mass: 42.65% of anhydrous calcium chloride, 41.5% of distilled water, 14.85% of glycerol and 1% of barium hydroxide octahydrate. The phase change temperature of the composite phase change material provided by the invention is 11.8 DEG C, the supercooling degree is 1.2 DEG C, the phase change enthalpy value is 112.86 J / g, and the phase change process is reversible and can be repeatedly used. The preparation method of the material comprises the following steps: weighing anhydrous calcium chloride and distilled water with corresponding mass, oscillating by using an ultrasonic oscillator until no obvious solid particles exist in the liquid to obtain an intermediate, sealing the intermediate by using a preservative film, standing to room temperature, adding glycerol and barium hydroxide octahydrate with corresponding mass into the intermediate, and uniformly stirring to obtain the material. And finally, stirring for 10 minutes by using a constant-temperature magnetic stirrer, and then packaging.
Owner:BEIBU GULF UNIV
A phase change material with a phase change temperature of -25°C
ActiveCN103265931BLarge latent heat of phase changeImprove performanceChemical industryHeat-exchange elementsSide effectCold storage
The invention discloses a phase-change material with a phase-change temperature of -25 DEG C. The phase-change material comprises, by weight, 5 to 25% of ammonium chloride, 4.5 to 25% of acrylic acid, 5 to 15% of alum, 5 to 25% of maleic anhydride, 5 to 10% of carboxymethyl cellulose sodium and 10 to 20% of grease, with the balance being water. According to the invention, the phase-change material with the phase-change temperature of -25 DEG C has the advantages of great latent heat of phase change, stable performances, no phase separation, reversible phase change process, capacity of being reused more than ten thousands of times, widely available raw materials, low cost, no toxic and side effects, safety, reliability, active effects of energy saving and emission reduction and capability of being popularized and applied in the processes of cold storage and refrigeration in fields like food and medicine.
Owner:SUZHOU ANTEK INDAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com