Inorganic phase-change constant-temperature material and preparation method thereof
An inorganic phase change and constant temperature technology, applied in the direction of heat exchange materials, chemical instruments and methods, can solve problems such as degradation of thermophysical properties, and achieve the effects of good plasticity, simple process flow, and easy scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
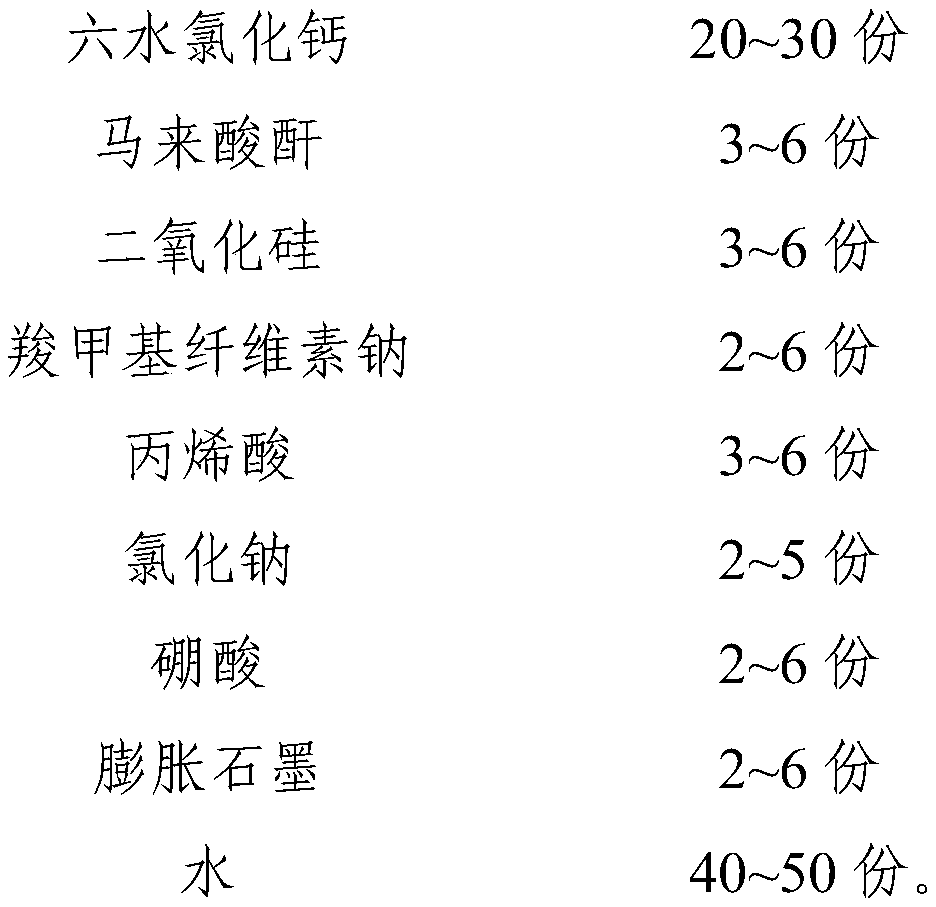
[0035] This embodiment provides an inorganic phase change constant temperature material, including the following components by weight:
[0036]
[0037]
[0038] The preparation method of the inorganic phase change constant temperature material is as follows:
[0039] (1) After mixing sodium carboxymethylcellulose and 10% water at 25-35°C, add maleic anhydride to obtain mixed solution I;
[0040] (2) Add silicon dioxide to the mixed solution I, then add 18% water, and react at 40° C. for 1 hour; obtain mixed solution II
[0041] (3) After mixing acrylic acid and 9% water, add sodium chloride to obtain mixed solution III;
[0042] (4) adding calcium chloride hexahydrate after mixing boric acid, expanded graphite, the mixed solution II and the mixed solution III, and reacting at normal pressure and 40° C. for 1 hour to obtain a polymer material;
[0043] (5) Mix the polymer material with the remaining part of water, and leave it for 10-15 minutes to obtain it.
[0044] ...
Embodiment 2
[0046] This embodiment provides an inorganic phase change constant temperature material, including the following components by weight:
[0047]
[0048]
[0049] The preparation method of the inorganic phase change constant temperature material is as follows:
[0050] (1) After mixing sodium carboxymethylcellulose and 9% water at 25-35°C, add maleic anhydride to obtain mixed solution I;
[0051] (2) Add silicon dioxide to the mixed solution I, then add 17% water, and react at 40° C. for 1 hour to obtain the mixed solution II;
[0052] (3) After mixing acrylic acid and 8% water, add sodium chloride to obtain mixed solution III;
[0053] (4) adding calcium chloride hexahydrate after mixing boric acid, expanded graphite, the mixed solution II and the mixed solution III, and reacting at normal pressure and 40° C. for 1 hour to obtain a polymer material;
[0054] (5) Mix the polymer material with the remaining part of water, and leave it for 10-15 minutes to obtain it.
[...
Embodiment 3
[0057] This embodiment provides an inorganic phase change constant temperature material, including the following components by weight:
[0058]
[0059]
[0060] The preparation method of the inorganic phase change constant temperature material is as follows:
[0061] (1) After mixing sodium carboxymethylcellulose and 8% water at 25-35°C, add maleic anhydride to obtain mixed solution I;
[0062] (2) Add silicon dioxide to the mixed solution I, then add 20% water, and react at 40° C. for 1 hour to obtain the mixed solution II;
[0063] (3) After mixing acrylic acid and 8% water, add sodium chloride to obtain mixed solution III;
[0064] (4) adding calcium chloride hexahydrate after mixing boric acid, expanded graphite, the mixed solution II and the mixed solution III, and reacting at normal pressure and 40° C. for 1 hour to obtain a polymer material;
[0065](5) Mix the polymer material with the remaining part of water, and leave it for 10-15 minutes to obtain it.
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Phase transition temperature | aaaaa | aaaaa |
| Latent heat of phase change | aaaaa | aaaaa |
| Latent heat of phase change | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com