Phase change energy storage material for heat preservation at night and preparation method
A technology of phase-change energy storage materials and nucleating agents, which is applied in the direction of heat exchange materials, chemical instruments and methods, etc., can solve the problems of high melting temperature of phase-change materials, inability to melt, and narrow solidification temperature range of phase-change materials. Achieve the effects of wide adjustable range, improved stability, and low melting temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 A phase change energy storage material that can be used for night heat preservation, the medium is composed of CaCl 2 -MgCl 2 -H 2 O water salt system and 1.0g nucleating agent borax.
[0031] Among them: CaCl 2 -MgCl 2 -H 2 The O water salt system consists of 95.0g calcium chloride hexahydrate, 1.3g magnesium chloride hexahydrate, and 3.7g deionized water.
[0032] The preparation method of the phase change energy storage material comprises the following steps:
[0033] (1) Weigh each raw material according to the ratio;
[0034] (2) Heating the recrystallized and purified calcium chloride hexahydrate in a constant temperature water bath at 40-50°C until completely melted to obtain a calcium chloride hexahydrate solution;
[0035] (3) Put the calcium chloride hexahydrate solution into a container, add magnesium chloride hexahydrate and deionized water under the condition of magnetic stirring with a rotating speed of 500~800r / min, and heat during th...
Embodiment 2
[0039] Example 2 A phase change energy storage material that can be used for night heat preservation, the medium is composed of CaCl 2 -MgCl 2 -H 2 O water salt system and 1.0g nucleating agent borax.
[0040] Among them: CaCl 2 -MgCl 2 -H 2 The O water salt system consists of 92.2g calcium chloride hexahydrate, 2.3g magnesium chloride hexahydrate, and 5.5g deionized water.
[0041] The preparation method of the phase change energy storage material is the same as Example 1 .
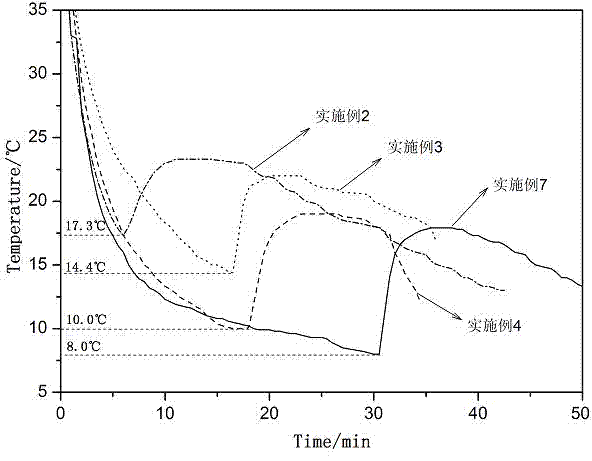
[0042] The obtained phase change material was tested by T-history method, and its melting temperature was 23.5-24.5°C, and its solidification temperature was 17.3°C.
Embodiment 3
[0043] Example 3 A phase change energy storage material that can be used for night heat preservation, the medium is composed of CaCl 2 -MgCl 2 -H 2 O water salt system and 1.0g nucleating agent strontium chloride hexahydrate composed.
[0044] Among them: CaCl 2 -MgCl 2 -H 2 The O water salt system consists of 90.7g calcium chloride hexahydrate, 2.8g magnesium chloride hexahydrate, and 6.5g deionized water.
[0045] The preparation method of the phase change energy storage material is the same as Example 1 .
[0046] The obtained phase change material was tested by T-history method, and its melting temperature was 23.0-23.5°C, and its solidification temperature was 14.4°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| phase transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com