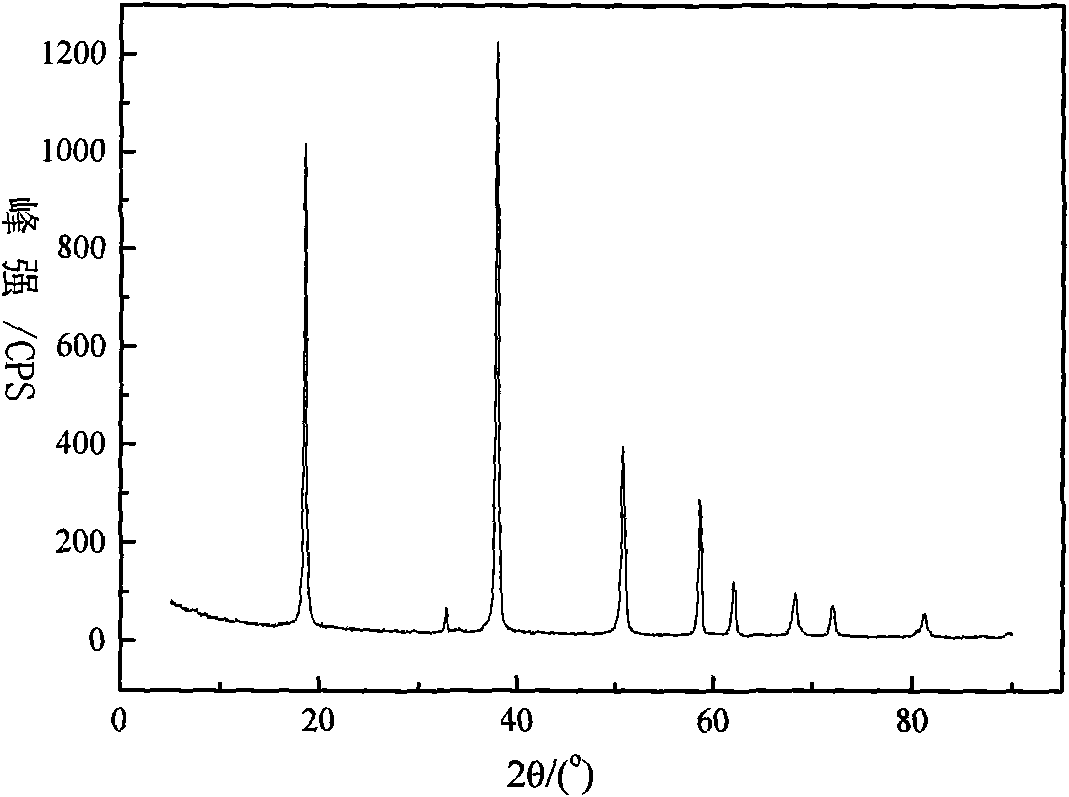
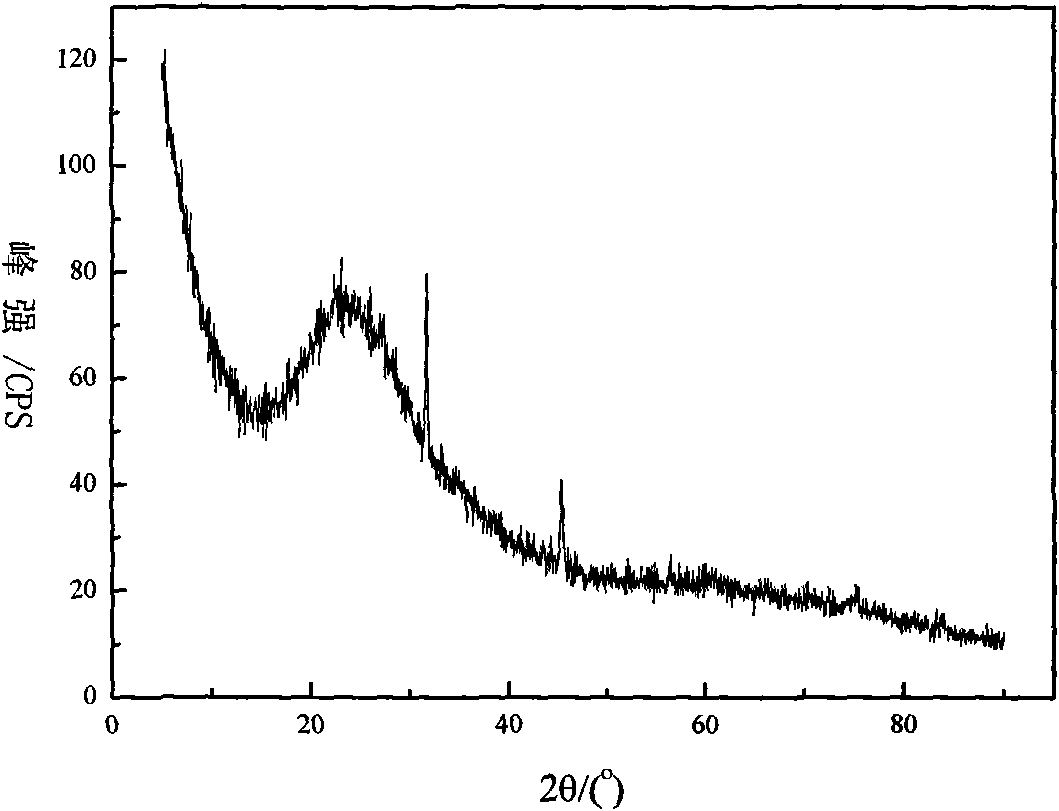
Method for preparing highly pure magnesium hydroxide and magnesium hexa-silicate by using olivine tailing mine
A technology of magnesium hydroxide and magnesium hexasilicate, applied in the directions of magnesium hydroxide, magnesium silicate, silicate, etc., can solve the problems of olivine powder having no economic value, incapable of direct industrial application, and low resource utilization rate, etc. Achieve the effect of reducing preparation cost, easy operation and high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
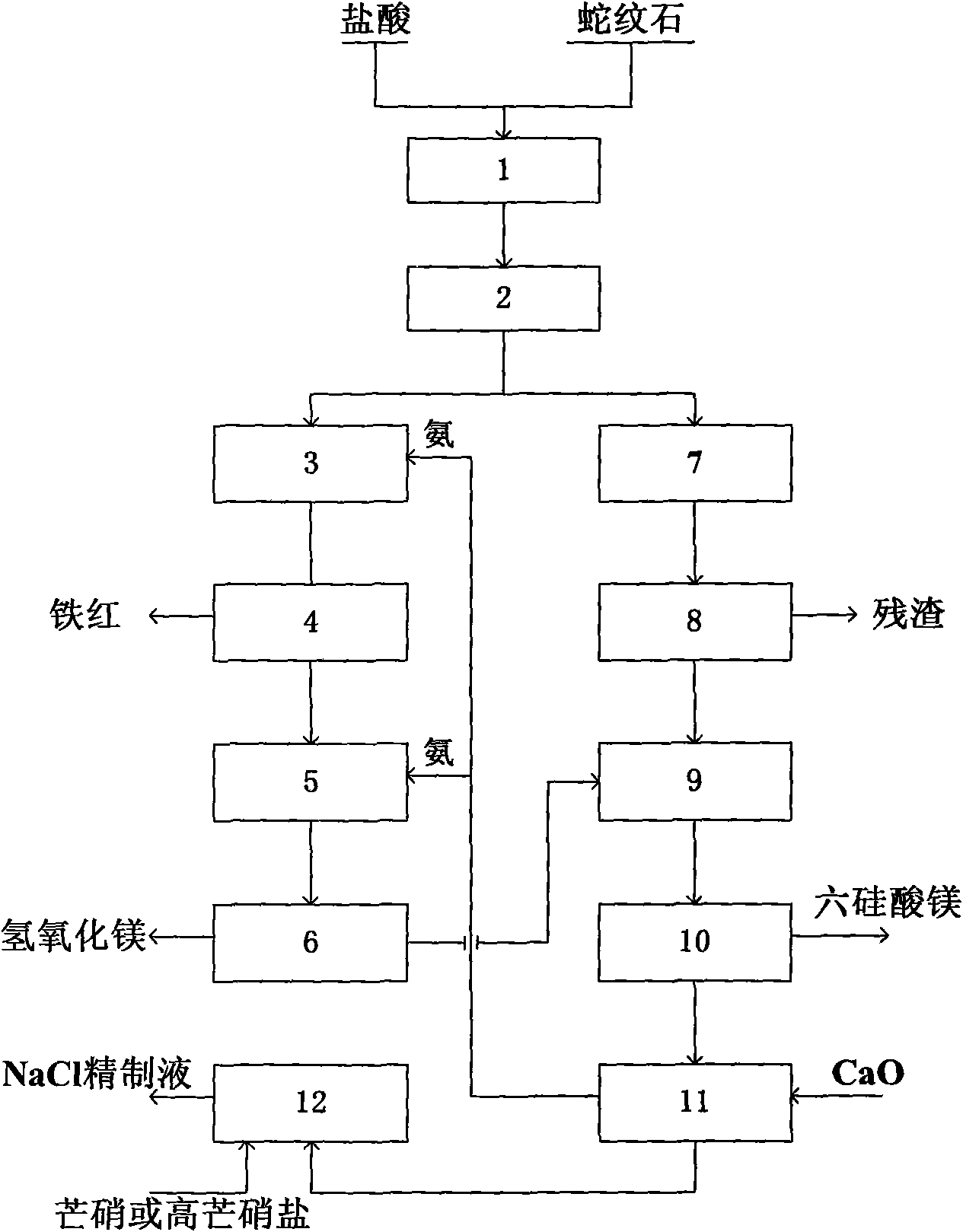
[0035] A method for preparing high-purity magnesium hydroxide and magnesium hexasilicate by utilizing olivine tailings, the steps are:
[0036] 1. Hydrochloric acid leaching 1: Pulverize the olivine powder to 90-110 mesh, put 100 grams of olivine powder into a 2L three-necked flask, add 750ml of hydrochloric acid solution (dilute 200ml of 36-38% hydrochloric acid to 750ml). The flask was placed in an oil bath, heated and stirred, and the reaction time was controlled to be 2 hours. A condenser was installed at the mouth of the bottle to ensure that the hydrochloric acid did not volatilize, and the temperature was controlled at 105°C. The final control pH is equal to 1. Its reaction equation is:
[0037] MgO+2HCl→MgCl 2 +H 2 o
[0038] Fe 2 o 3 +6HCl→2FeCl 3 +H 2 o
[0039] FeO+2HCl→FeCl 2 +H 2 o
[0040] 2. Filtration 2: vacuum filter the liquid and solid mixture after hydrochloric acid treatment, and wash the filter cake with 100 ml of water. Ammonia and heavy iro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com