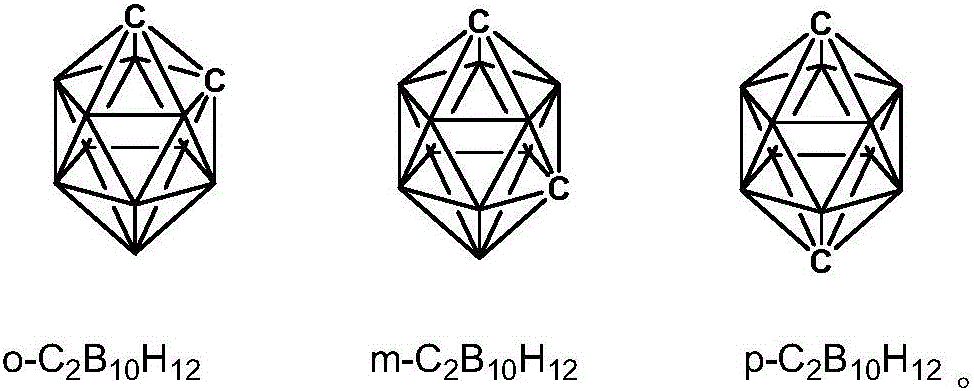Diamine monomer containing carborane and preparation method thereof
A technology of diamine monomer and carborane is applied in the field of carborane structure-containing diamine monomer and its preparation, and the preparation of polyimide materials for high temperature resistance and neutron radiation protection. Description, unfavorable large-scale synthesis, no mention of neutron protection and other issues, to achieve the effects of low generation of side reactions and impurities, low incidence of side reactions, and high practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
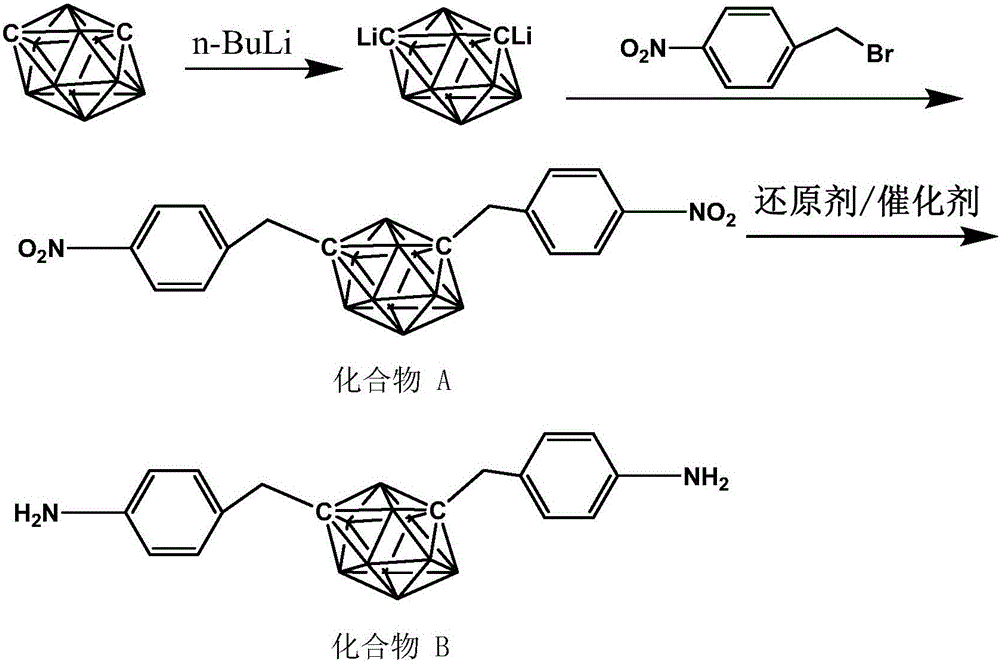
[0028] In a 150ml three-necked flask equipped with magnetic stirring, add 1.4668g (10mmol) m-carborane (m-C 2 B 10 H 12 ), the reaction system is maintained at -10°C, anhydrous, oxygen-free, and argon atmosphere. Add 20ml of tetrahydrofuran (THF) treated with molecular sieves, and wait until m-C 2 B 10 H 12 After being completely dissolved in THF, slowly add 6.56ml (2.5M) n-BuLi solution (n-BuLi) dropwise through a constant pressure dropping funnel, the system is a pale white suspension, react for 5h to lithiation of carborane Complete, cool down to -35°C. Weigh 4.342g (20.1mmol) of p-nitrobenzyl bromide, dissolve it in 20ml THF, add dropwise to the carborane double lithium salt solution through a dropping funnel, react at low temperature for 3 hours, then react at room temperature for 3 hours, then The temperature was raised to reflux for 10 hours to complete the reaction. Quench with 5ml 1M hydrochloric acid, extract with ethyl acetate (20ml*3 times), wash with saturated brin...
Embodiment 2
[0030] The preparation process of carborane bisnitro compound A is the same as in Example 1. Add 3g of compound A to a three-necked flask (150ml) with nitrogen gas, add 0.3g of catalyst Pd / C and 50ml of absolute ethanol, and heat to 70°C , 10ml of reducing agent ammonium formate was added dropwise to the reaction system, stirred and heated to reflux for 5h, and the reaction was complete. After the Pd / C was filtered out, the remaining solution was dried by rotary evaporation to obtain the carborane diamine monomer, namely compound B The yield of this step is 92%.
Embodiment 3
[0032] The preparation process of carborane bisnitro compound A is the same as in Example 1. Add 3g of compound A to a three-necked flask (150ml) with nitrogen gas, add 0.3g of catalyst Pd / C and 50ml of absolute ethanol, and heat to 70°C , Pass hydrogen into the reaction system, stir and heat to reflux for 5 hours, and then the reaction is complete. After the Pd / C is filtered out, the remaining solution is dried by rotary evaporation to obtain the carborane diamine monomer, namely compound B. The yield of this step Is 90%.
[0033] FT-IR(KBr,cm -1 ):2932(C-H), 2526(B-H), 1470(CH 2 ), 1017(B-B).
[0034] 1 HNMR(400MHz,DMSO-d 6 ,ppm)δ:7.40(d,4H,Ar-H), 7.10(d,4H,Ar-H), 5.58(s,4H,NH 2 ), 2.87-1.40 (m, 14H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com