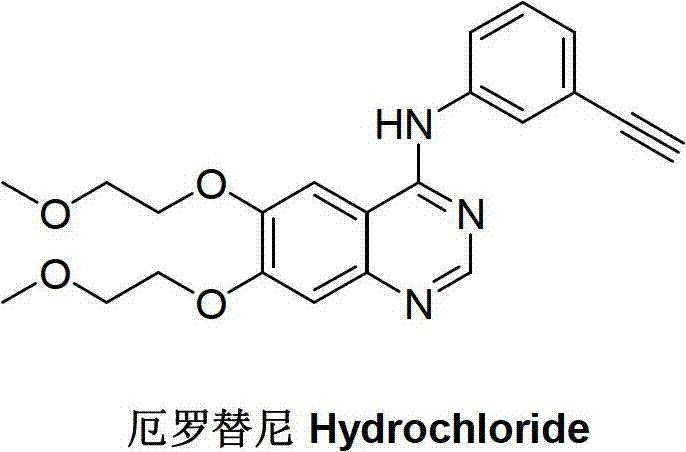
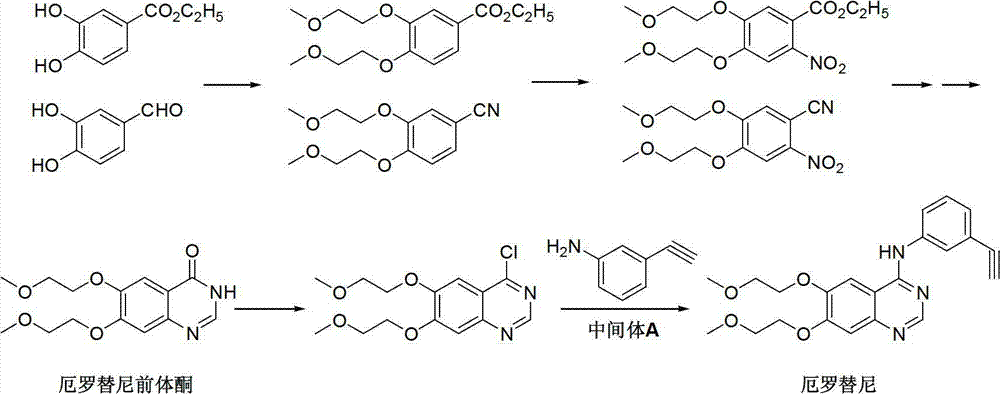
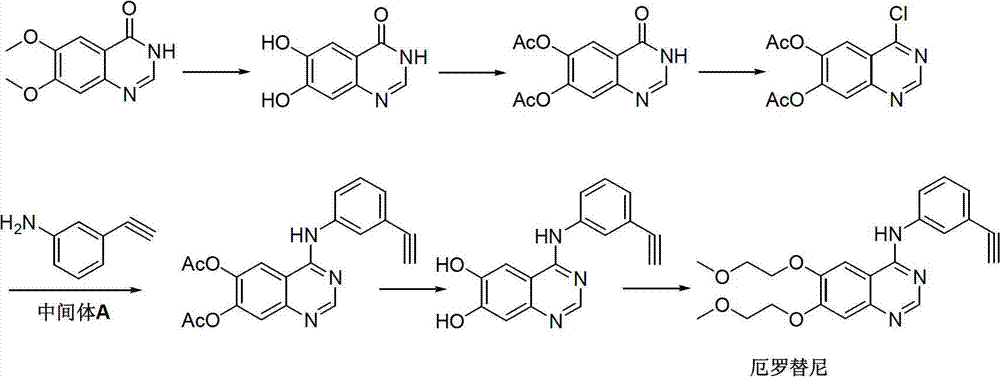
Preparation method of erlotinib intermediate, i.e., 3-aminobenzeneacetylene
A technology of aminophenylacetylene and erlotinib, which is applied in the field of preparation of erlotinib intermediates, can solve the problems of high cost and low yield, and achieve the effects of reducing manufacturing costs, promoting development, and improving atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The technical solution of the present invention is described in further non-limiting detail below.
[0033] A method for preparing erlotinib intermediate 3-aminophenylacetylene, the chemical formula of the erlotinib intermediate 3-aminophenylacetylene is as follows, hereinafter referred to as intermediate A;
[0034]
[0035] The method comprises the steps of: using formula I economically available industrial raw material 3-nitroacetophenone, obtaining the intermediate 1-chloride containing halogen-substituted ethylenic bond shown in formula II through Wellsmeier (Vilsmeier) reaction -1-(3-nitrophenyl)-2-(N,N-dimethylmethoxime)-yl-ethylene, the intermediate is hydrolyzed to obtain the aldehyde intermediate 1-chloro- 1-(3-nitrophenyl)-2-formaldehyde)-base-ethylene, the aldehyde intermediate of formula III is through elimination reaction, obtains the intermediate 3-nitrophenylacetylene of formula IV; The intermediate 3 of formula IV -Nitrophenylacetylene is passed thr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com