Synthetic method of mirabegron
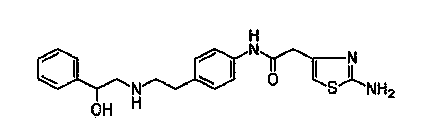
A synthesis method and condensation reaction technology are applied in the synthesis field of mirabegron, which can solve the problems of high production cost, three wastes, irritation and the like, and achieve the effects of high product yield, low cost and cost reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
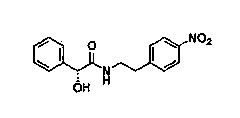
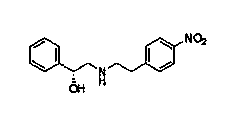
[0015] The invention discloses a synthesis method of Mirabegron, which comprises the following steps: a. Amino transesterification: using (R)-mandelic acid ester and 4-nitrophenethylamine salt as raw materials to carry out transesterification reaction, Obtain Mirabegron intermediate product A; b, reduction amide: the amide reduction of described Mirabegron intermediate product A, obtain Mirabegron intermediate product B; C, reduction nitro: the described Mirabegron Intermediate product B carries out reduction reaction with reductant tin protochloride, obtains Mirabegron intermediate product C; D, condensation reaction: described Mirabegron intermediate product C and 2-aminothiazole-4-acetic acid are in condensation agent Under the effect of condensation reaction, Mirabegron is obtained.
[0016] Preferably, the step a specifically includes the following steps: dissolving (R)-mandelic acid ester in solvent 1, and sequentially adding 4-nitrophenethylamine salt and an organic bas...
Embodiment l
[0022] A kind of synthetic method of Mirabegron, the method comprises the following steps:
[0023] a. Amino transesterification: Add 5.90 kg of 4-nitrophenethylamine salt, (R)-ethyl mandelate, and 2.94 kg of organic base triethylamine in sequence and dissolve them in 35L 1,2-xylene solvent. Stir for 1 hour, reflux for 5 hours, and use high-performance liquid phase analysis to detect the reaction degree of the reaction raw materials. After the reaction is completed, distill 1,2-xylene under pressure, distill 15 L, cool down, stir overnight, precipitate solids, filter, and cool the Obtain 8.34 kg of Mirabegron intermediate product A after washing and drying with 1,2-xylene; the chemical structural formula of the Mirabegron intermediate product A is as follows:
[0024]
[0025] b. Reducing amide: Dissolve 1.6 kg of anhydrous lithium chloride in 36 L of anhydrous tetrahydrofuran, add 1.44 kg of sodium borohydride, stir at room temperature for two hours, and then add 6 kg of m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com