Aromatic azoxybenzene compound and preparation method thereof
A technology for oxidizing azobenzene and compounds, which is applied in the preparation of nitro compounds, the preparation of organic compounds, and the production of hydrocarbons from oxygen-containing organic compounds, etc., can solve the problems of high reaction temperature feed ratio, complicated catalyst preparation, environmental pollution and the like, To achieve the effect of efficient reaction, low cost and simple components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The synthesis of embodiment one aromatic nitro base
[0068] Synthesis of Compound A:
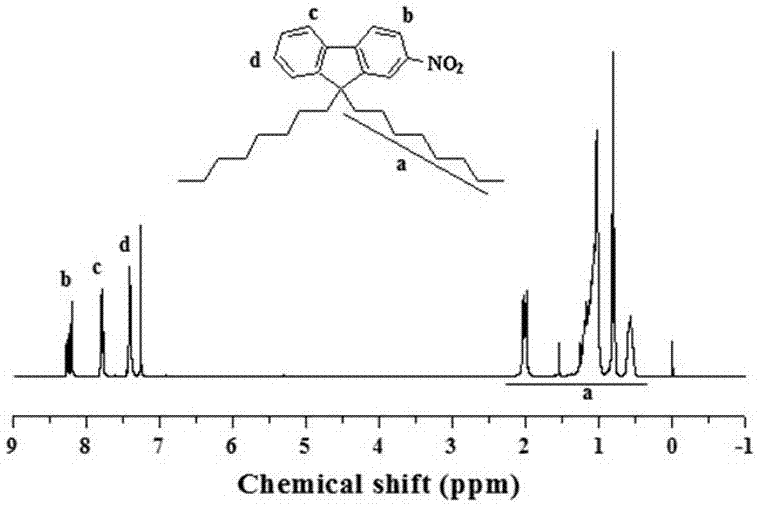
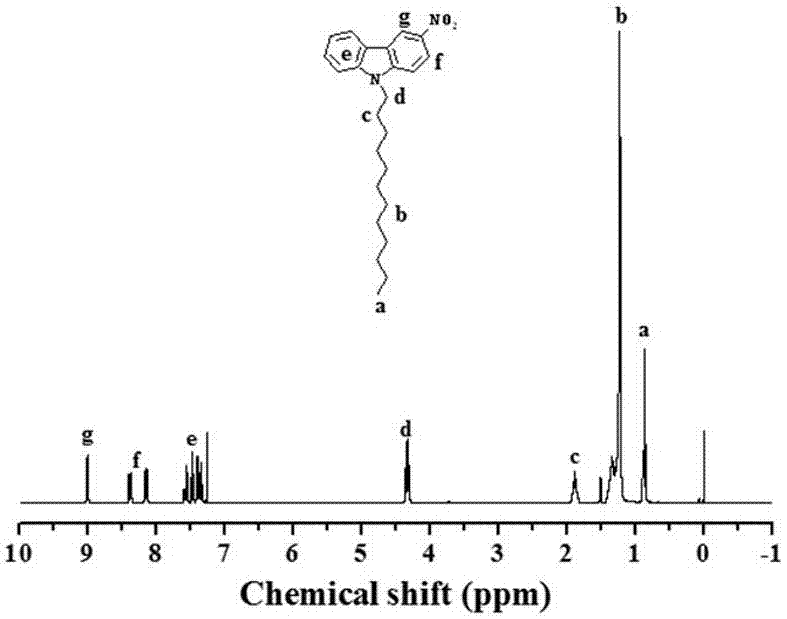
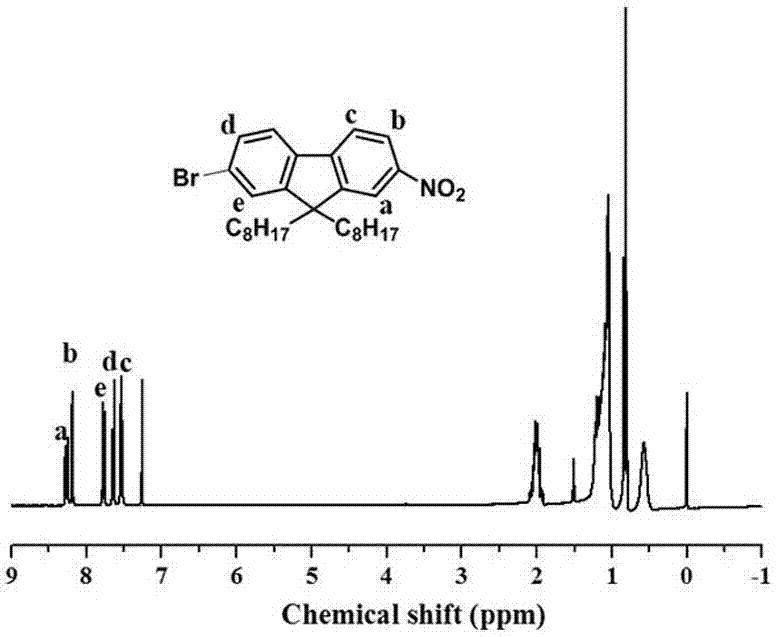
[0069] Add 80 mL of 1,2-dichloroethane to a 250 mL flask, then add 20 mL of concentrated nitric acid (65%) to make it evenly dispersed, react in an ice-water bath, then slowly add 10 g of fluorene, and react for 20 min. It was poured into 300 mL ice methanol, and a light yellow solid 2 (11 g) was obtained after suction filtration. Grind 7.5 g KOH into powder and add it to 100 mL DMSO, disperse evenly, then slowly add 6.3 g of the above-mentioned light yellow solid, stir for 20 min, then add 15 mL 1-bromooctane dropwise to the above system, react for 30 min, It was extracted with petroleum ether, dried, and spin-dried to obtain a crude product, which was then subjected to column chromatography using petroleum ether as a developing solvent to obtain a yellow viscous liquid, Compound A (9.0 g).
[0070] Synthesis of Compound B:
Embodiment 2
[0086] The preparation of embodiment two aromatic azobenzene compounds
[0087] Add 11.2 mg potassium hydroxide, 0.1 mmol aromatic nitro base (compound A, compound B, compound C, compound D, compound E or compound F), 3 mL toluene and 3 mL isopropanol to a 10 mL ampoule successively, After the addition, argon was blown for 10 minutes to deoxygenate and the tube was sealed, and then reacted for 10 hours under the irradiation of a xenon lamp (power 250-1000 W is optional). After the reaction was completed, it was extracted with dichloromethane and dried over anhydrous sodium sulfate. , spin-dried, and then purified by column chromatography (developing solvent: petroleum ether / dichloromethane) to obtain the corresponding aromatic azobenzene oxide compound.
[0088] attached Figure 7-12 The NMR spectra of aromatic azobenzene oxide compounds prepared by Compound A, Compound B, Compound C, Compound D, Compound E, and Compound F respectively, and the yields of the corresponding produ...
Embodiment 3
[0089] Example Preparation of triaromatic azobenzene compounds (under sunlight)
[0090] Add 11.2 mg of potassium hydroxide, 0.1 mmol of compound A, 3 mL of toluene and 3 mL of isopropanol to a 10 mL ampoule successively. After the addition is complete, deoxygenate and seal the tube with argon gas for 10 min, and then store in sunlight at room temperature Reacted for 20 h; after the reaction, extracted with dichloromethane, dried over anhydrous sodium sulfate, spin-dried, and then purified by column chromatography (developing solvent: petroleum ether / dichloromethane=20 / 1-8 / 1) to obtain The corresponding aromatic azobenzene compound has a yield of 93%.
[0091] The process flow diagram of above-mentioned preparation aromatic nitro base is as follows:
[0092] .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com