Synthesis method of 6,7-substituted-4-aniline quinazoline
A synthetic method and substituent technology, applied in 6 fields, can solve the problems of expensive raw materials, complicated reaction steps, unstable intermediate products, etc., and achieve the effect of reducing production costs and improving competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0034] In order to make the above and other objects, features and advantages of the present invention more comprehensible, the following will be described in detail with the accompanying drawings.
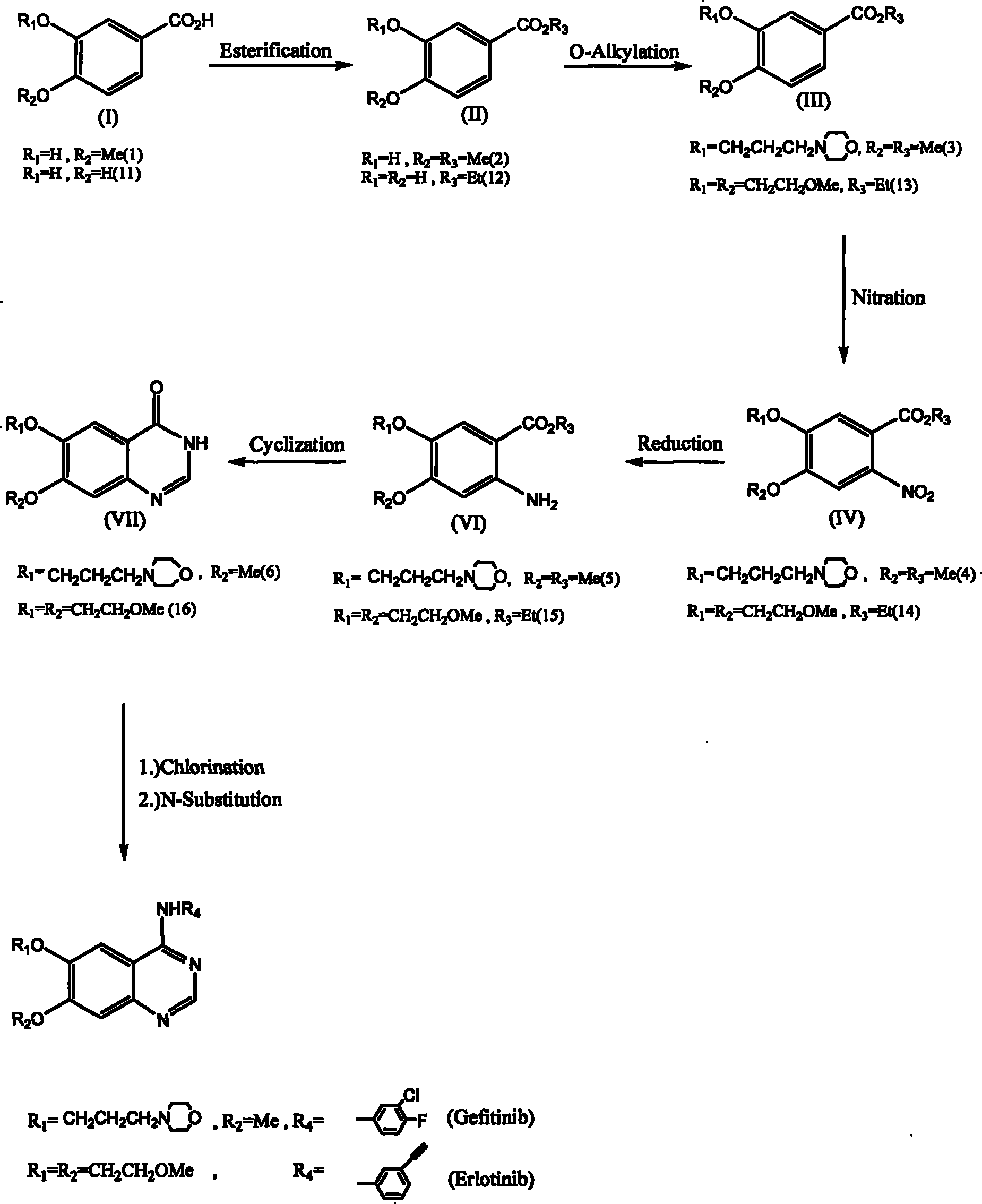
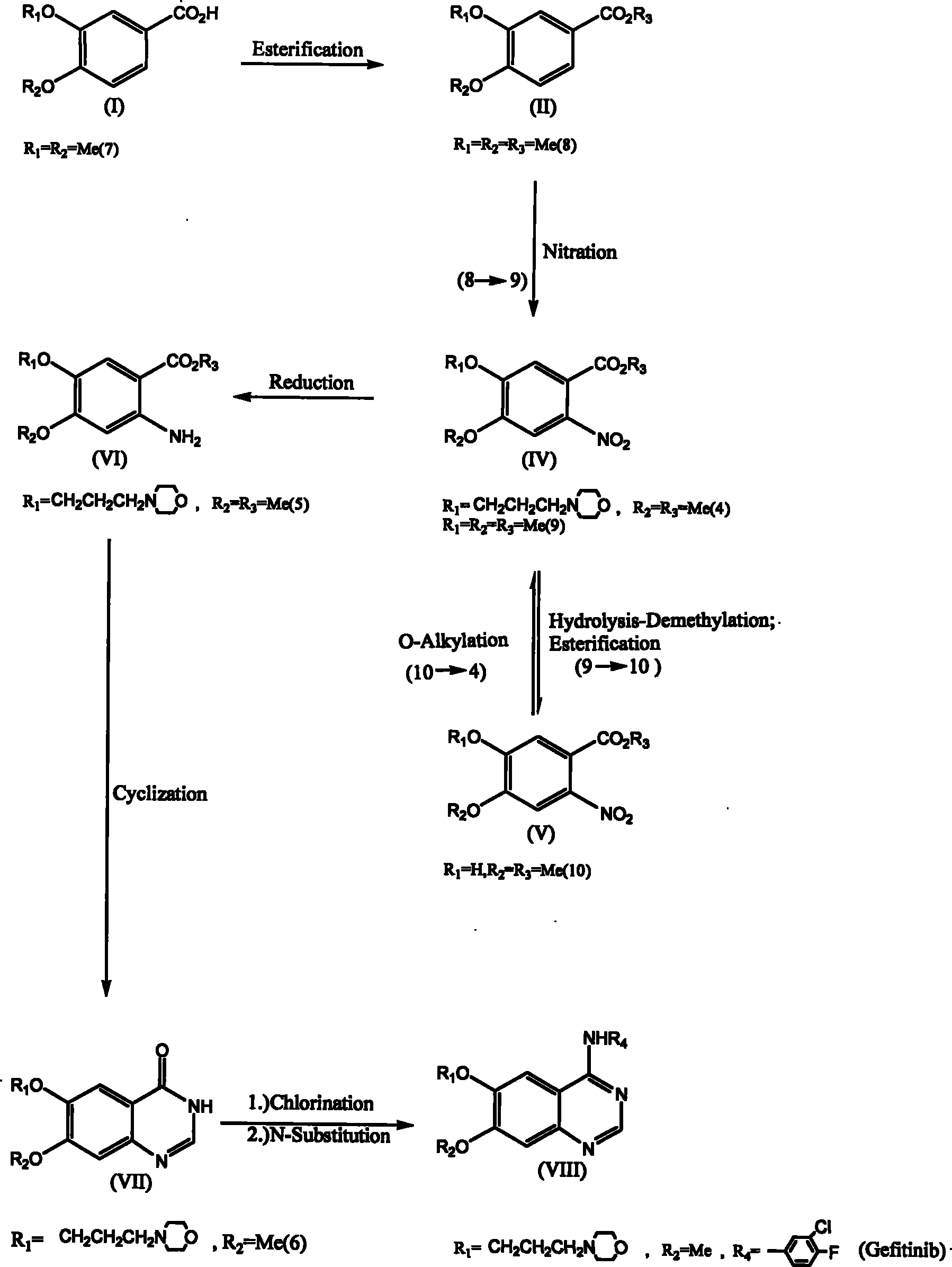
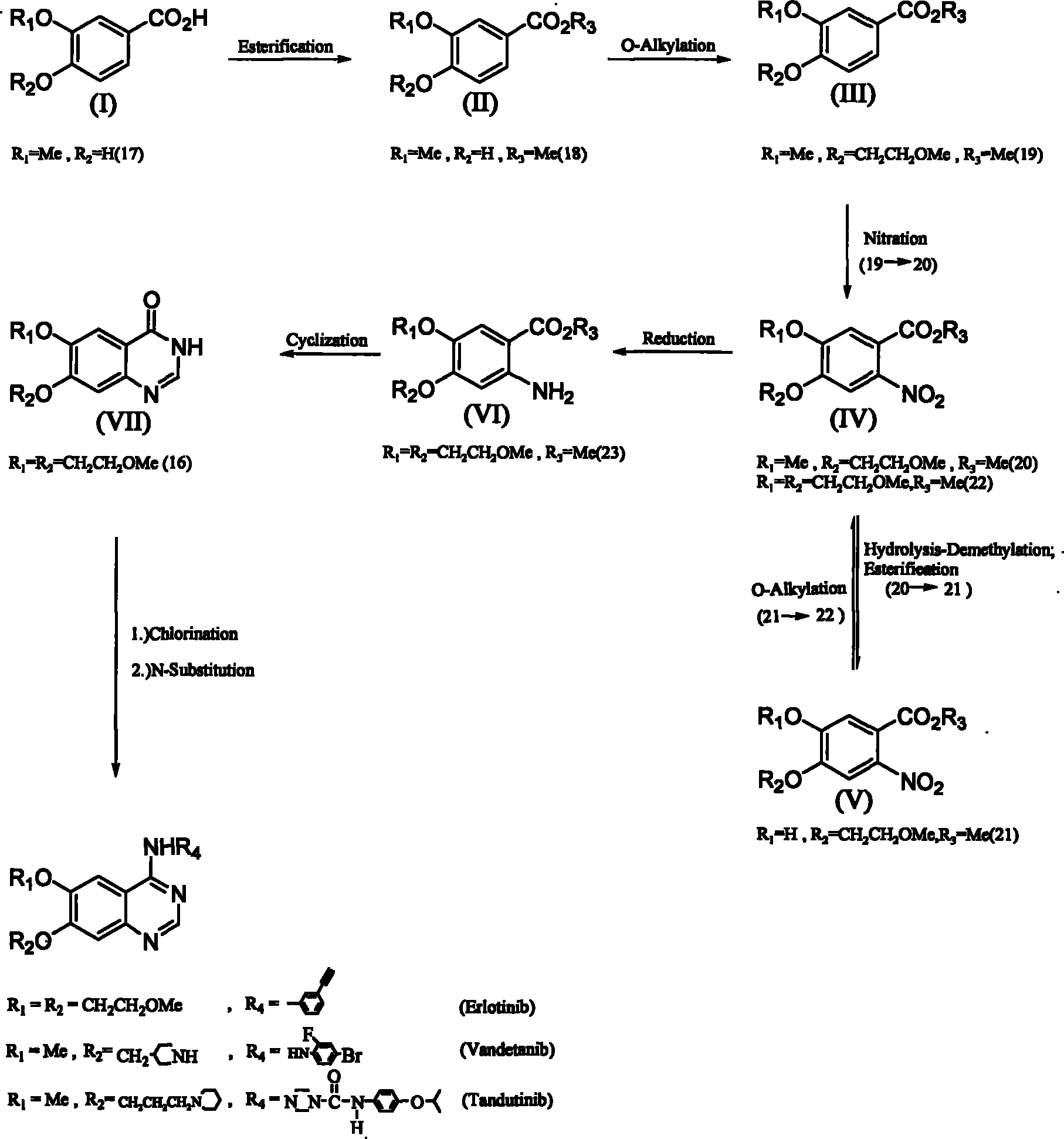
[0035] The invention discloses a method for synthesizing 6,7-substituent-4-aniline quinazolines using 3,4-substituted benzoic acid (3,4-substituted benzoic acid) as a starting material; wherein, a synthesis method It can be prepared through esterification reaction, oxygen-alkylation reaction, nitration reaction, nitro reduction reaction, cyclization reaction and two-in-one reaction (one-pot reaction) without going through the hydrolysis-demethylation reaction step. Obtain 6,7-substituting group-4-aniline quinazoline; Another synthetic route must go through hydrolysis-demethylation reaction, and its synthesis step comprises esterification reaction, oxygen-alkylation reaction or nitration reaction, hydrolysis -Demethylation reaction, esterification reaction, oxygen-alkylation reactio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com