Novel method for preparing Eltrombopag intermediate
A compound and a defined technology, applied in the field of preparation of Eltrombopag intermediates, can solve problems such as difficult handling, high production costs, and inability to apply mechanically, and achieve the effects of reducing three wastes emissions, reducing material costs, and ingenious design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063]
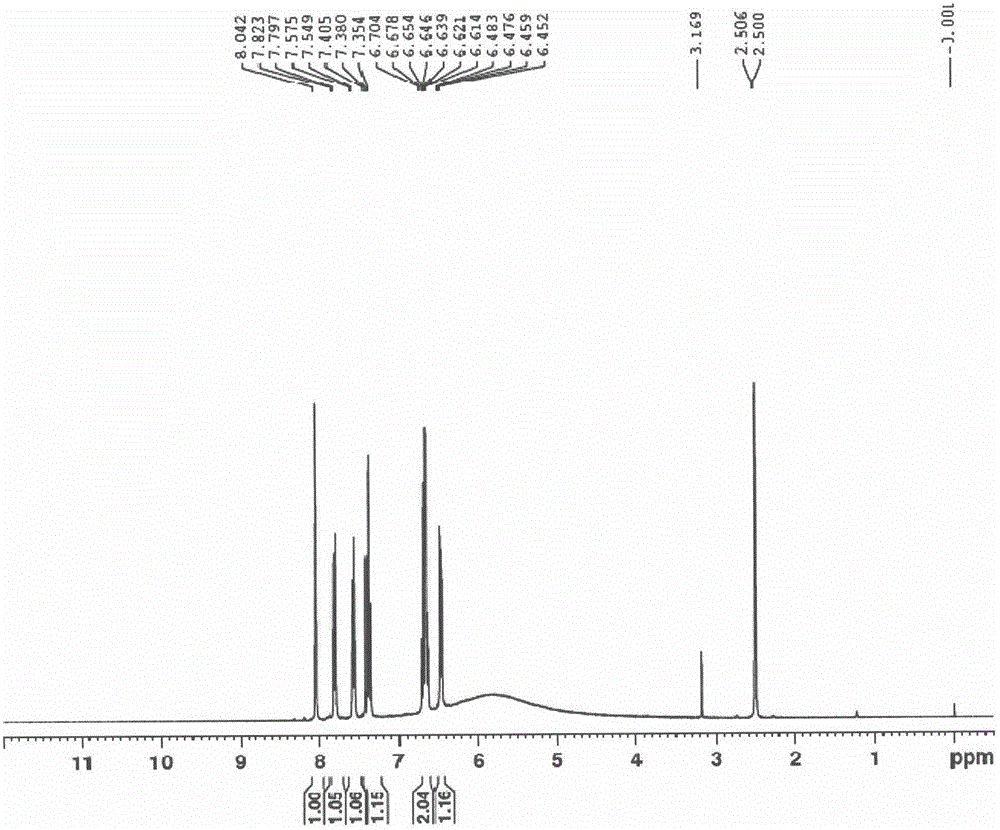
[0064] Acetic acid (7.5L) and 4-chloro-2-bromophenol (1500.0g, 7.2mol) were added successively in the reaction flask and stirred thoroughly; sodium nitrate (1229.0g, 14.5mol) and concentrated sulfuric acid (1418.0g, 14.5mol) were added dropwise at room temperature ) aqueous solution (1.4L); after dropping, control the temperature at 25-30°C and react for about 20-30min; stop the reaction, pour the reaction solution into ice water (7.5L), and stir thoroughly for 20min; filter with suction, and wash the filter cake with water Until the filtrate is nearly neutral (PH 6-7); the filter cake is dried to obtain a yellow solid: 1742g, yield: 95%. mp 122~124℃; 1 H NMR (400MHz, DMSO) δ8.11(d, J=2.6Hz, 1H), 8.05(d, J=2.6Hz, 1H); ESI-MS(m / z): 250[M-H] - .
Embodiment 2
[0066]
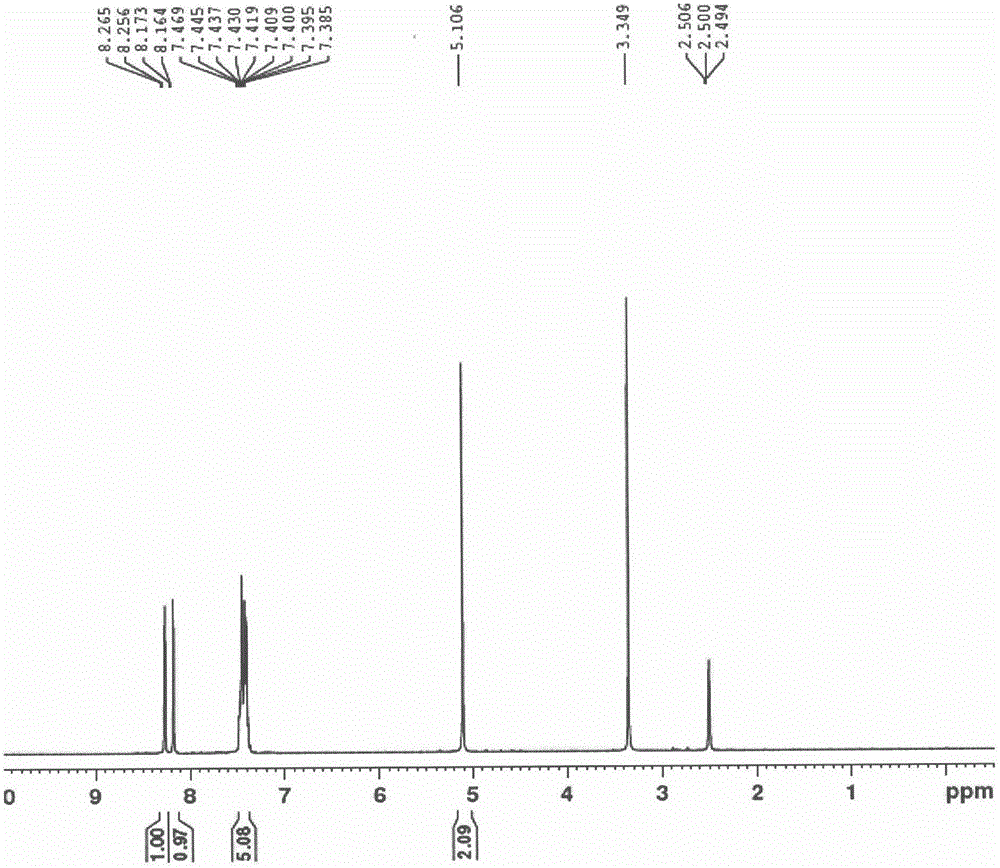
[0067] In the reaction flask, add DMF (2.5L) successively, compound II (500.0g, 1.98mol) potassium carbonate (821.2g, 5.9mol), fully stir; dropwise add benzyl bromide (508.1g, 3.0mol), dropwise process without Obvious exothermic phenomenon; after dripping, react at 20-25°C for 4-6h, pour the reaction solution into water (12.5L), stir well, filter with suction, rinse the filter cake with water, and dry the filter cake to obtain a light yellow solid: 598g, Yield 88%. mp 74~76℃; 1 H NMR (300MHz, DMSO) δ8.26 (d, J=2.7Hz, 1H), 8.16 (d, J=2.7Hz, 1H), 7.39-7.47 (m, 5H), 5.11 (s, 2H).
Embodiment 3
[0069]
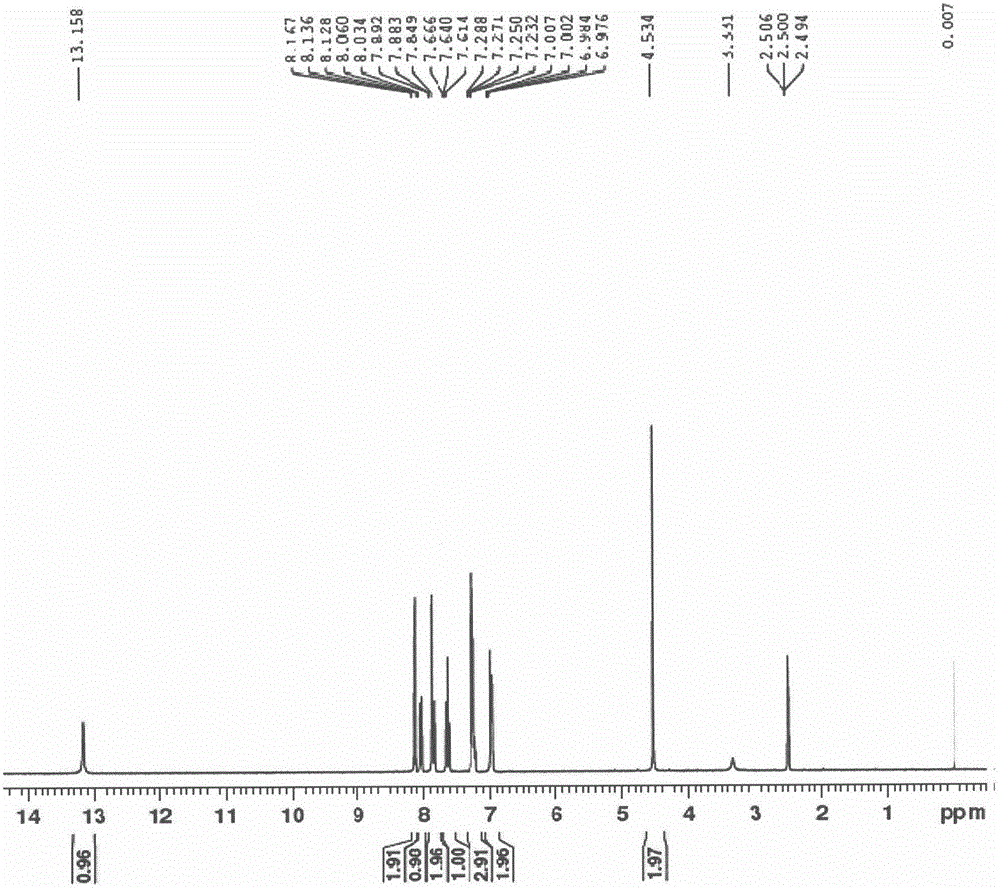
[0070] In the reaction flask, add compound III-1 (500.0g, 1.46mol), methanol (2.5L), sodium carbonate (309.0g, 2.91mol), water (2.5L), 3-carboxyphenylboronic acid (242.0g, 1.46mol), 10% palladium carbon (50.0g), nitrogen replacement three times, reflux reaction for 24h. Stop the reaction, suction filter while it is hot, rinse the palladium-carbon filter cake with hot methanol, and recover the palladium-carbon. Combine the filtrates, control the temperature below 20°C, add concentrated hydrochloric acid dropwise to adjust to acidity (pH=1-2); filter with suction, rinse the filter cake with water, wash the filter cake with methanol / ethyl acetate (V / V, 1 / 2) Beat, filter, and dry the filter cake to obtain a light yellow solid: 487g, yield: 87%, purity 99.14% [HPLC method: chromatographic column ODS-C 18 (150*4.6mm, 5um); column temperature 35°C; flow rate 1mL / min; mobile phase A: 10mM sodium dihydrogen phosphate (PH=3), flow B: acetonitrile (gradient elution: 0→10min,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com