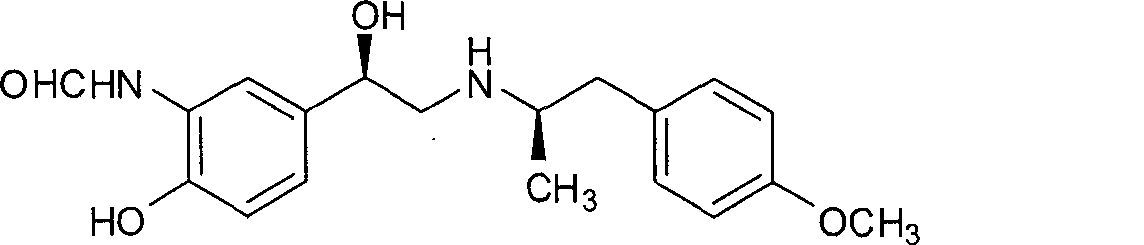
Asymmetric synthesis method of (R,R)-formoterol tartrate
A technology of formoterol and synthesis method, applied in the field of chiral β2-adrenoceptor agonist synthesis, can solve the problems of low utilization rate of raw materials, complex synthesis, increased cost, etc., and achieve good yield and diastereomeric selection performance, the process route is simple, and the effect of reducing the cost of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
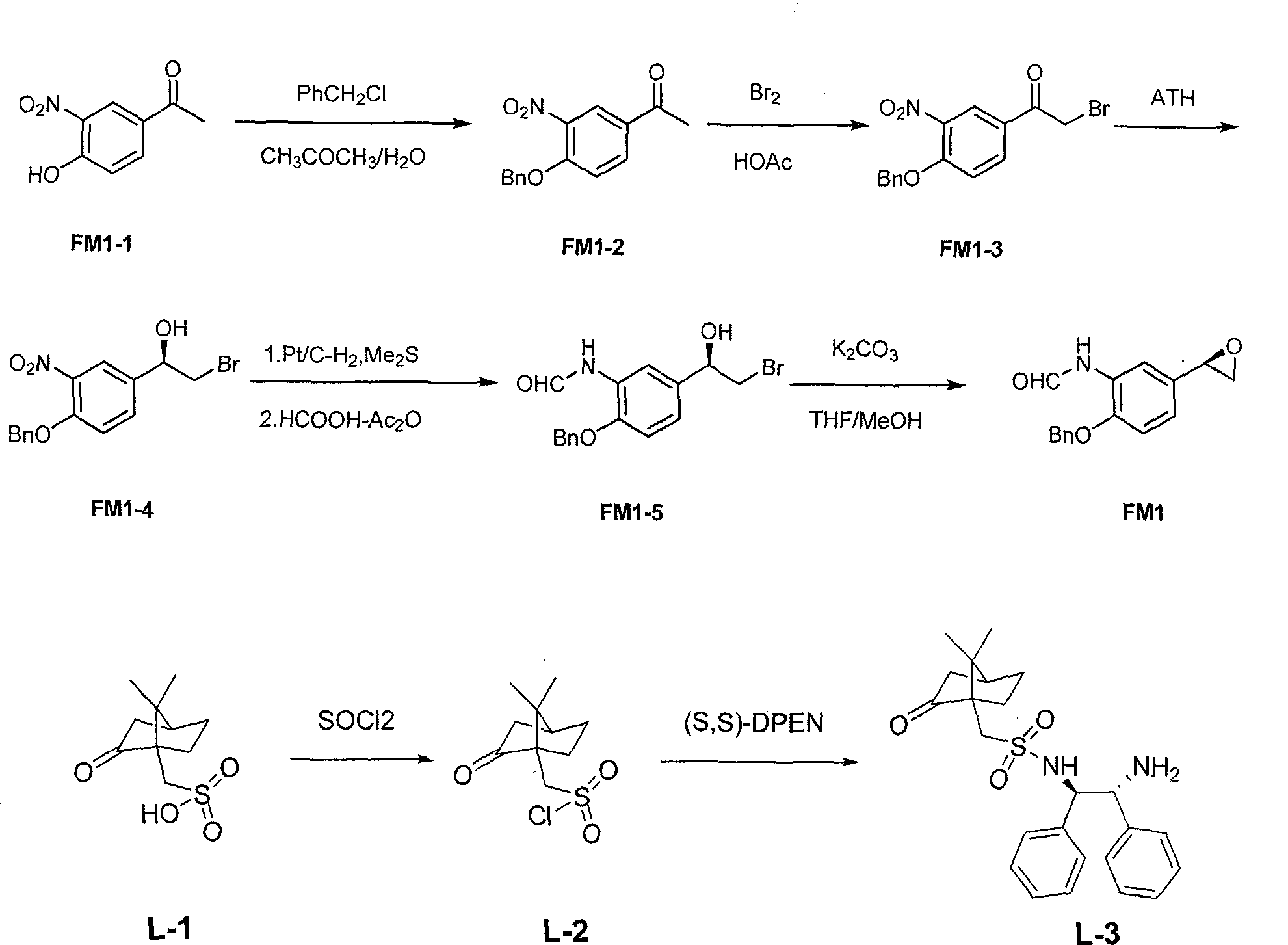
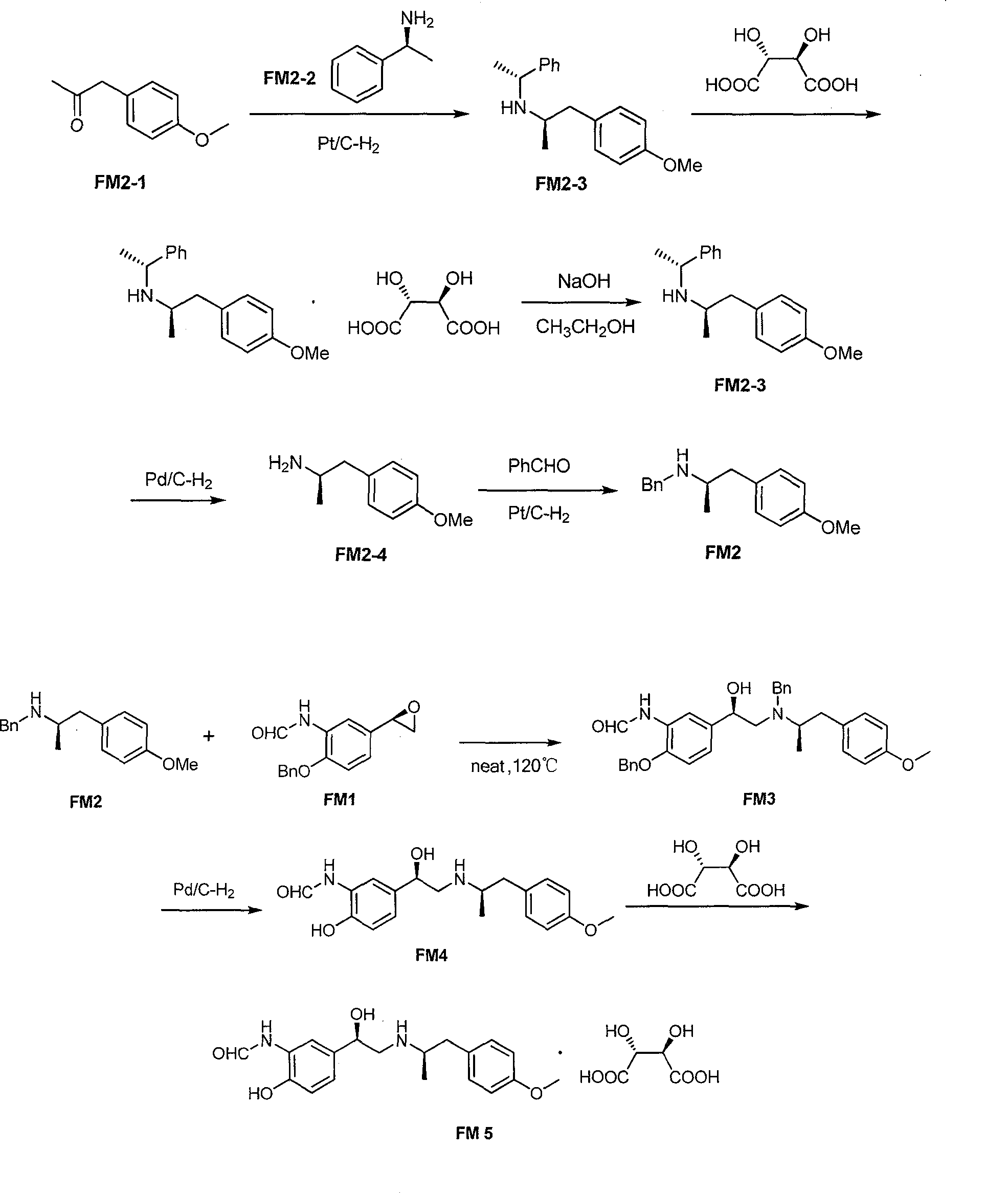
[0040] The first step: the preparation of 4-benzyloxy-3-nitroacetophenone (FM1-2)
[0041] Add 103.5 g (0.75 mol) of potassium carbonate and 600 ml of water into a 2 L reaction flask, and stir until completely dissolved. Then add 90.5g (0.5mol) of 4-nitro-3-hydroxyphenylethanone (FM1-1), stir for 0.5h, add 600ml of acetone, stir for 0.5h, add 16.6g (0.01mol) of potassium iodide and benzyl chloride 88.2 g (0.7 mol), heated to reflux, kept at reflux for 16 hours, TLC tracking confirmed the end of the reaction. After the reaction was completed, the reaction system was cooled to room temperature, filtered with suction, and the filter cake was washed with water (1000ml×3) and dried. 138.0 g (97.8%) of light yellow solid powder FM1-2 was obtained, mp135-138°C.
[0042] The second step: the preparation of 1-(4-(benzyloxy)-3-nitrophenyl)-2-bromoethanone (FM1-3)
[0043] Add 54.26g (0.40mol) of FM1-2 and 560ml of glacial acetic acid into a 2L reaction flask, stir at room temperature...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com