Composite ion exchanger, preparation method and applications thereof
A composite ion and exchanger technology, applied in the direction of ion exchange, cation exchange, cation exchange materials, etc., can solve the problems of separation difficulty, low reuse rate, difficult recovery, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] The preparation of embodiment 1 composite ion exchanger, Cs + and Sr 2+ Ion removal rate test
[0100] Preparation of sample 1#;
[0101] Containing [Sn 3 S 7 ] n 2n- The selection of layered sulfides with anion framework [(CH 3 ) 2 NH 2 ] 4 / 3 [(CH 3 ) 3 NH] 2 / 3 sn 3 S 7 1.25H 2O, synthesized according to existing reports (CN104399538A, J. Mater. Chem. A, 2015, 3, 5665-5673).
[0102] In the examples, unless otherwise specified, containing [Sn 3 S 7 ] n 2n- The layered sulfide and layered sulfide of the anion skeleton both refer to [(CH 3 ) 2 NH 2 ] 4 / 3 [(CH 3 ) 3 NH] 2 / 3 sn 3 S 7 1.25H 2 O.
[0103] Take 1.0g particle size less than 100 mesh containing [Sn 3 S 7 ] n 2n- Dissolve the layered sulfide of the anionic skeleton in 6mL dimethyl sulfoxide, and stir for 30min at a speed of 400rpm; then add 0.4g polyacrylonitrile and continue heating to 40°C to make the solution viscous; These viscous liquids were added dropwise to a large amou...
Embodiment 2
[0115] The structural characterization of embodiment 2 sample
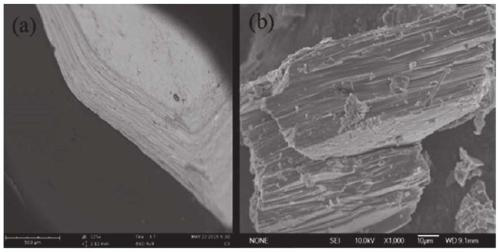
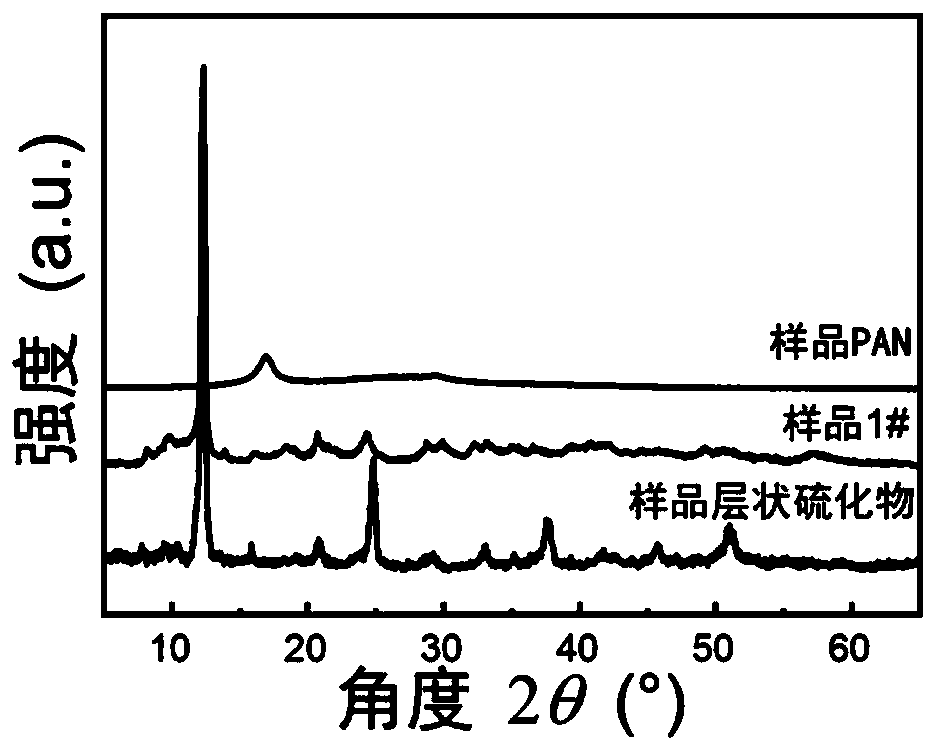
[0116] Containing [Sn 3 S 7 ] n 2n- The layered sulfides with anion framework, sample PAN and sample 1# were characterized by field emission scanning electron microscopy (FE-SEM) and X-ray powder diffraction phase analysis (XRD).
[0117] figure 2 (a) Field emission scanning electron microscope (FE-SEM) image of the sample layered sulfide; figure 2 (b) is the field emission scanning electron microscope (FE-SEM) image of sample 1#. Field emission scanning electron microscopy (FE-SEM) characterization results showed that sample 1# maintained the layered structure of sulfide, and the surface was evenly distributed with PAN particles, and these PAN particles were in a dispersed state without obvious aggregation. Therefore, it can be concluded that polyacrylonitrile and layered sulfide compound to form layered sulfide / PAN spherical composite ion exchanger does not change the phase structure of polyacrylonitrile...
Embodiment 3
[0119] Example 3 Sample 1# removes Cs + and / or Sr 2+ Ionic performance test
[0120] Take 18mL of a solution containing 3000ppm cesium chloride, then add 18mg of sample 1#, the mixture is stirred at room temperature for 10h, then the mixture is centrifuged to get the supernatant and the initial solution to determine its Cs by atomic absorption spectrometry or plasma emission spectrometry + Concentrations before and after exchange. The ion exchange product was filtered and washed thoroughly with distilled water, ethanol and acetone in sequence to obtain Cs + The product after ion exchange is denoted as sample 1#—Cs.
[0121] Take 18mL of a solution containing 1000ppm strontium chloride, then add 18mg of sample 1#, the mixture is stirred at room temperature for 10h, and then the mixture is centrifuged to take the supernatant and the initial solution to determine its Sr by atomic absorption spectrometry or plasma emission spectrometry. 2+ Concentrations before and after excha...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com