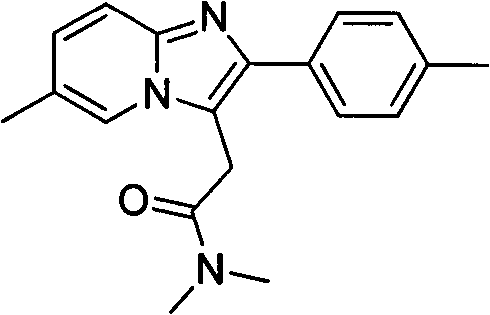
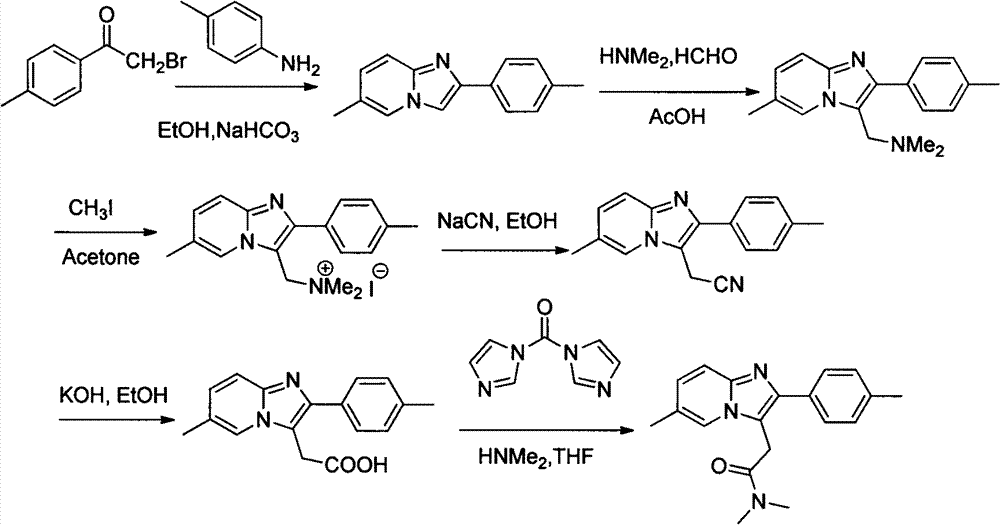
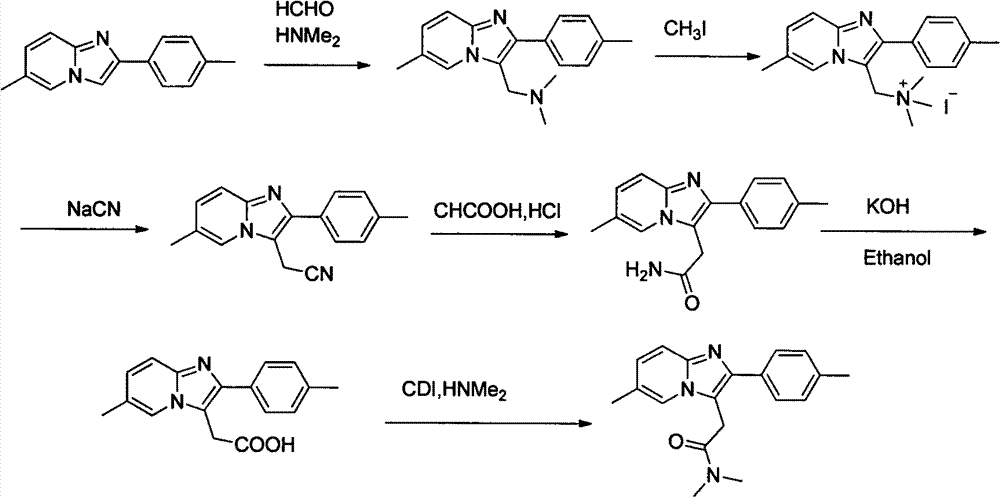
Method for preparing compound zolpidem
A compound, zolpidem technology, applied in the field of chemical synthesis, can solve the problems of unreported zolpidem synthesis, inability to synthesize zolpidem, unsuitable for industrial production, etc., and achieve the effect of short reaction steps, low cost and atom economy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Preparation of 5-methyl-N-(4-methylbenzylidene)pyridin-2-amine
[0048] Add 395-methyl-2-aminopyridine, 260mg p-toluenesulfonic acid hydrate and 80ml anhydrous toluene into a dry 250ml two-necked bottle, stir to dissolve, add 3.4ml 4-methylbenzaldehyde, under the water separator device Stir and reflux for 12 hours; then evaporate most of the toluene, cool down to 90-80°C, transfer the reaction solution to a dry 50ml round-bottomed flask, and distill under reduced pressure to obtain 3.8g of white solid with a yield of 65.2%.
[0049] ESI-MS: [M+H] = 211.0; 1 H NMR (400MHz, CDCl3) δ9.11(s, 1H), 8.29(s, 1H), 7.86(d, J=7.9Hz, 2H), 7.53(d, J=8.0Hz, 1H), 7.26(d , J=7.8Hz, 2H), 7.22(d, J=8.0Hz, 1H), 2.40(s, 3H), 2.33(s, 3H).
Embodiment 2
[0050] Example 2 Preparation of N, N, 6-trimethyl-2-(4-methylphenyl)-imidazo[1,2-a]pyridine-3-acetamide
[0051] In a dry 25ml reaction vial, add 10mgCul, 105mg5-methyl-N-(4-methylbenzylidene)pyridin-2-amine and 97mgN, N-dimethylpropynamide successively, then add 2ml without Water toluene, reflux reaction for 36h; after the reaction is completed, cool to room temperature, dilute with 5ml of dichloromethane, then add 6-7 drops of triethylamine, stir for 10min, filter under reduced pressure under a small amount of basic alumina, and filtrate The evaporator was concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography (petroleum ether: ethyl acetate: triethylamine-1:1:0.1) to obtain the target product, 111 mg of white solid, with a yield of 72%.
[0052] 1 H NMR (400MHz, CDCl3) δ8.02(s, 1H), 7.57(d, J=7.6Hz, 2H), 7.54(s, 1H), 7.28(d, J=6.2Hz, 2H), 7.06(d , J=9.2Hz, 1H), 4.11(s, 2H), 2.94(s, 3H), 2.90(s, 3H), 2.40(s, 3H), 2.3...
Embodiment 3
[0053] Example 3 Preparation of N, N, 6-trimethyl-2-(4-methylphenyl)-imidazo[1,2-a]pyridine-3-acetamide
[0054] In a dry 25ml reaction bottle, add 108mg 5-methyl-2 aminopyridine, 19mg p-toluenesulfonic acid hydrate, 5ml anhydrous toluene to dissolve, then add 124.3μl 4-methylbenzaldehyde, reflux for 6h, cool to room temperature, then add 194 mg N, N-dimethylpropynamide and 16 mg CuSO 4 , the temperature rose to 60° C., reacted for 4 hours, and ended the reaction; the post-treatment operation method was the same as in Example 2, and 40 mg of the target product was obtained with a yield of 13%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com