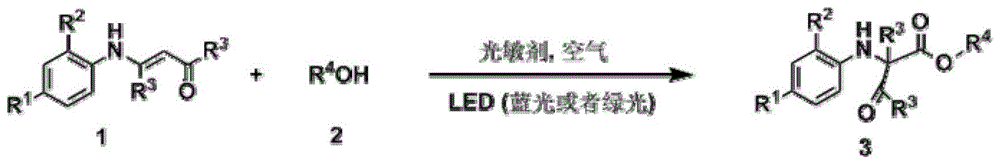
Visible light-catalyzed acyl transfer method for preparation of aniline derivatives
An aniline derivative and a technology for catalyzing acyl groups are applied in the field of catalytic synthesis to achieve the effects of mild synthesis reaction conditions, atom economy and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
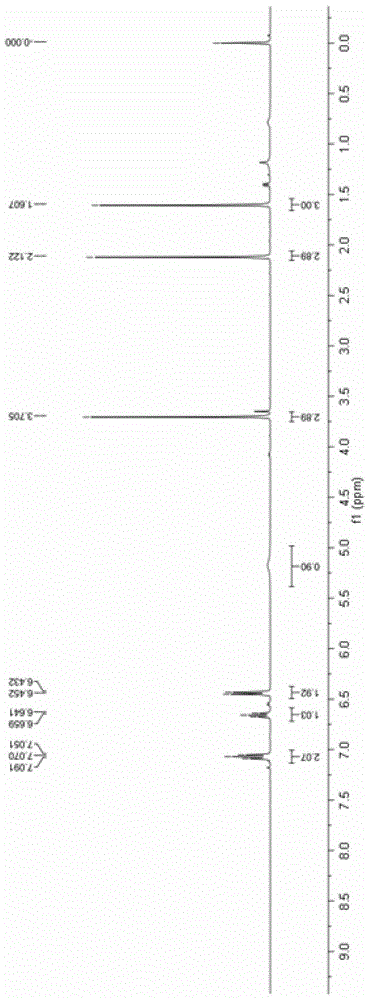
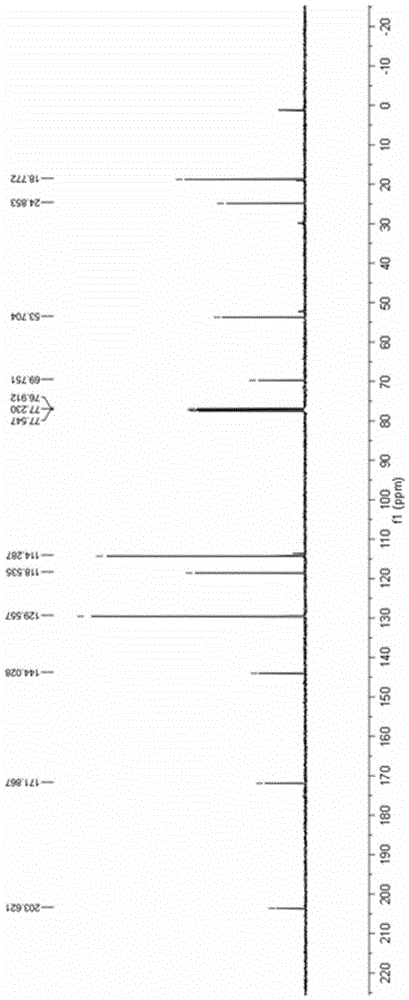
[0042] Add 0.2mmol 4-anilino-3-ene-2-pentanone and 0.002mmol ruthenium chloride coordinated by terpyridine to a 10mL reaction test tube, use 4mL methanol as the reaction solvent, and use LED (blue light) to illuminate the reaction solution in the air After 12 hours, the reaction solvent was spin-dried, and then separated by a column, and the yield of the product obtained was 88%. H NMR, C NMR, and mass spectrometry identified the product as 2-methyl-2-anilino-3-oxobutanoic acid methyl ester.
[0043] The product 2-methyl-2-anilino-3-oxobutanoic acid methyl ester ester hydrogen spectrogram and carbon spectrogram respectively see specification attached figure 1 and figure 2 .
Embodiment 2
[0045] Same as Example 1, the difference is that the selected photosensitizer is ruthenium hexafluorophosphate coordinated by terpyridine, and the yield of the product obtained is 87%.
Embodiment 3
[0047] With embodiment 1, the difference is that the solvent selected is ethanol, and the product yield obtained is 84%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com