Industrial production method of furan derivative
A technology of furan derivatives and furan rings, which is applied in organic chemistry and other fields, can solve the problems of long compound synthesis routes, uneconomical atoms, complex by-products, etc., and achieve the effects of green production process, low raw material cost and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
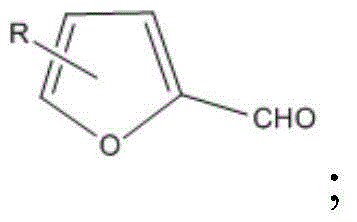
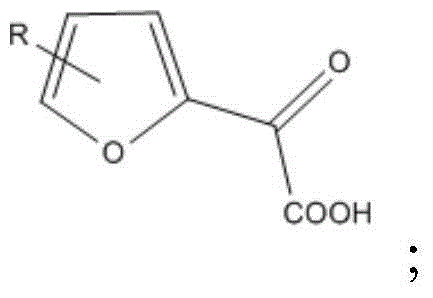
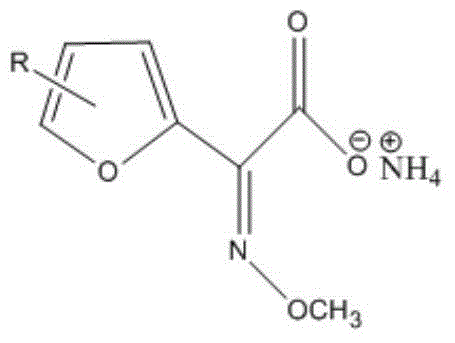
[0047] The reaction equation is as follows:
[0048]
[0049] Put 9600g of compound A into the reactor, add 2700g of HCN, stir and react at room temperature for 5 hours, then add 600g of nickel, feed air (or oxygen), and heat the system at a speed of 2-4°C / min while stirring to 60°C, react for 8 hours, and stop the reaction. (The reaction method with catalyst participation can also be used in the reaction process with HCN, such as: metal catalyst, biocatalyst, Lewis, etc.)
[0050] Cool to room temperature, add 1 L of water, stir for 1 hour, adjust the pH to weak acidity, filter, and recrystallize twice with a solvent of acetone:cyclohexane=1:1 to obtain 12999 g of product B.
[0051] Or, after the above-mentioned reaction is finished, after cooling to room temperature, add 4700g of methoxyamine, adjust to an appropriate pH value with hydrochloric acid, and stir the reaction for 2 hours at room temperature, detect that the HPLC raw material is <5%, and feed 1700g of ammoni...
Embodiment 2
[0057] The reaction equation is as follows:
[0058]
[0059] Put 3000ml of acetonitrile, 9600g of compound A into the reaction kettle, add 4900g of NaCN and 100g of ion exchange resin at 25°C, stir and react for 1.5 hours, cool to room temperature, filter off the resin, evaporate the solvent, add 1L of water, and stir for 3 hours , add 100g of nickel, feed air (or oxygen), heat the system to 60°C at a speed of 2-4°C / min while stirring, react for 6 hours, and stop the reaction.
[0060] Cool to room temperature, add 1 L of water, stir for 1 hour, adjust the pH to weak acidity, filter, and recrystallize twice with a solvent of acetone:cyclohexane=1:1 to obtain 13132 g of product B.
[0061] Or, after the above-mentioned reaction is completed, after cooling to room temperature, add 4700g of methoxyamine, adjust to an appropriate pH value with hydrochloric acid, and stir the reaction for 2 hours at room temperature, detect that the HPLC raw material is <5%, and feed 1700g of amm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com