Method for preparing p-hydroxy-cinnamate by using ionic liquid to catalyze lignin
A technology of ionic liquid and cinnamate, which is applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., can solve problems such as low efficiency and poor selectivity, and achieve simple preparation, atom economy, and realization of recycling Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
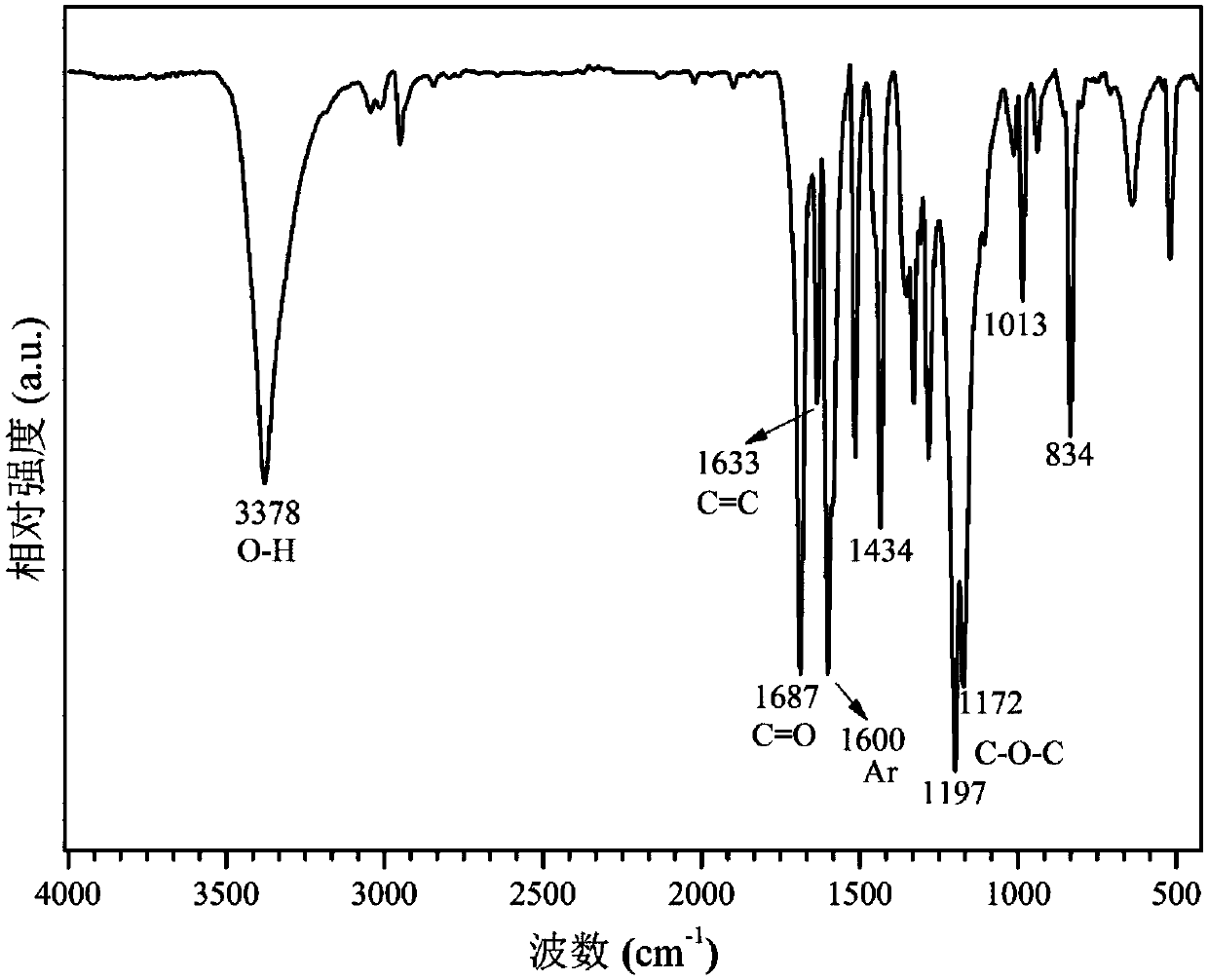
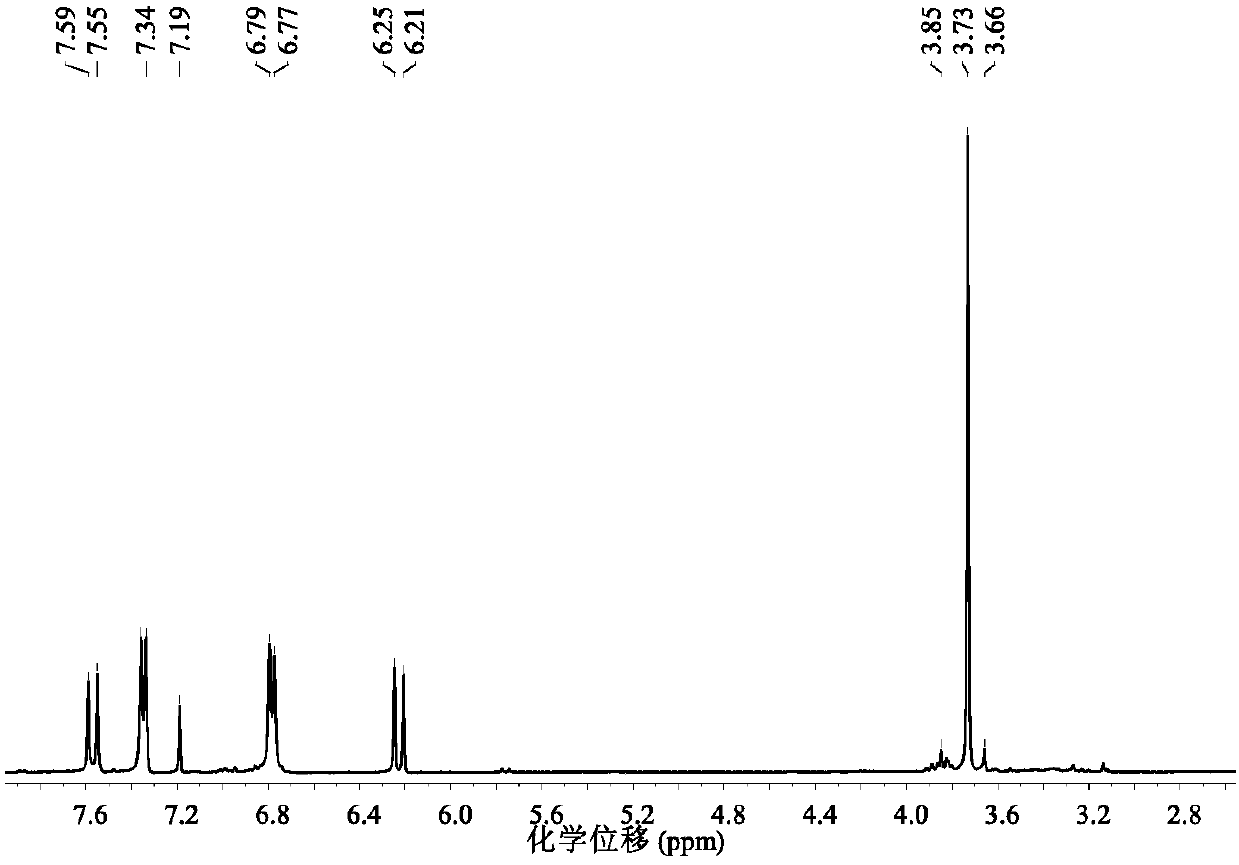
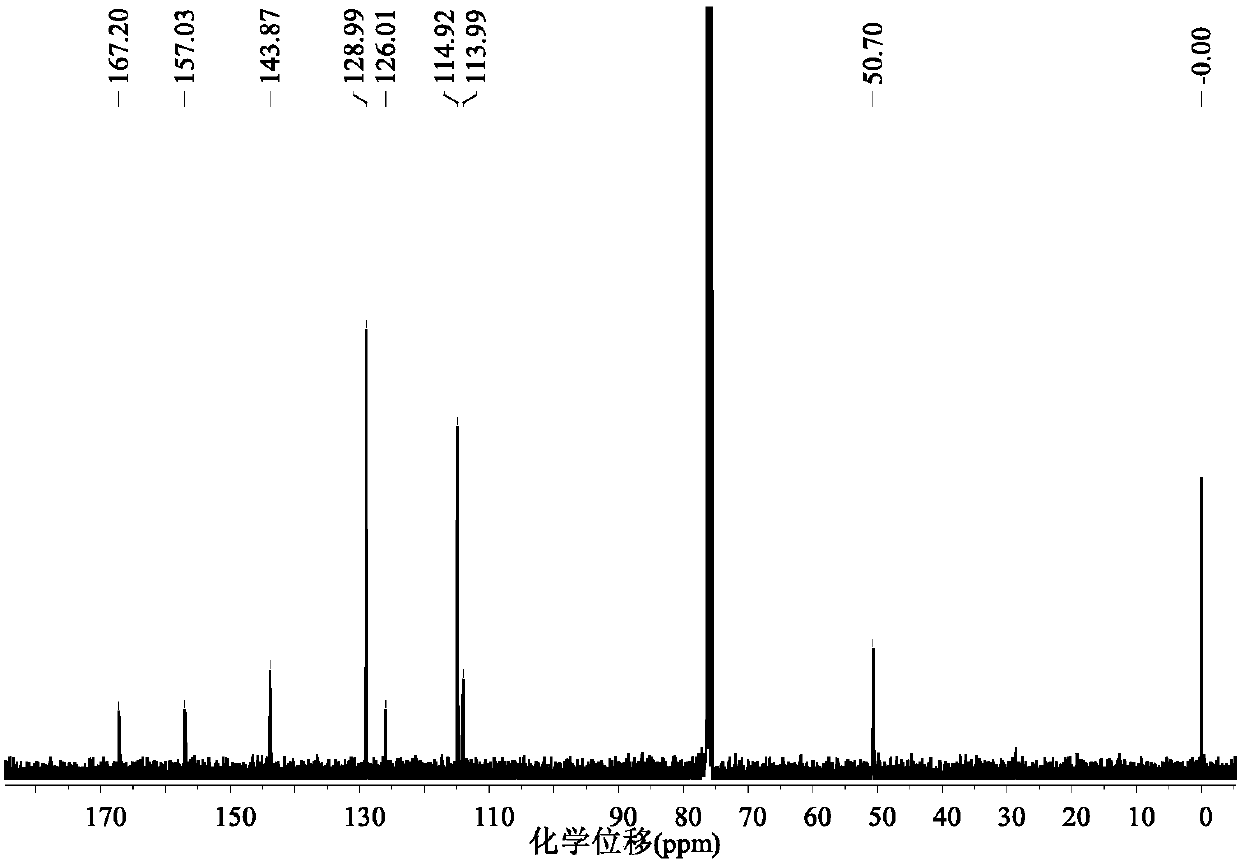
[0037] A method utilizing ionic liquid to catalyze lignin to prepare p-hydroxycinnamate, the operation steps are as follows:
[0038] (1) Halogen metal salt ionic liquid [C 4 mim][FeCl 4 ] Preparation: 1-butyl-4-methylimidazolium chloride salt C of 20mmol ferric chloride and equivalent substance amount 4mimCl was mixed, stirred at 30°C for 3h, extracted with 5mL of dichloromethane, the resulting solution was evaporated to remove the solvent, and dried in vacuum at 60°C for 12-24h to obtain the metal salt halogen ionic liquid [C 4 mim][FeCl 4 ].
[0039] (2) Extraction of organic lignin: add 250mL ethanol, 100mL deionized water, 1.0g sulfuric acid, and 20g bagasse powder into a 500mL stainless steel reaction kettle, react at 160°C for 4h, filter and separate, and add deionized water to the liquid phase. After the water is filtered and separated, the solid phase obtained is organic lignin.
[0040] (3) catalytic depolymerization of lignin: the gained halogen metal salt ioni...
Embodiment 2
[0042] The difference between this embodiment and embodiment 1 is:
[0043] (1) Halogen metal salt ionic liquid [C n mim][AlCl 4 ] preparation: 1-ethyl-4-methylimidazolium chloride salt C of 10mol aluminum chloride and equivalent substance quantity 2 mimCl was mixed, stirred at 80°C for 12h, extracted with 15mL of dichloromethane, the resulting solution was evaporated to remove the solvent, and vacuum-dried at 70°C for 12-24h to obtain the metal salt halogen ionic liquid [C 2 mim][AlCl 4 ].
[0044] (2) Extraction of organic lignin: add 200mL ethanol, 100mL deionized water, 1.5g sulfuric acid, and 30g corn stalk powder into a 1L stainless steel reaction kettle, react at 120°C for 6h, filter and separate, add the liquid phase After deionized water is filtered and separated, the obtained solid phase is organosoluble lignin.
[0045] (3) catalytic depolymerization of lignin: the gained halogen metal salt ionic liquid of step (1) [C 2 mim][AlCl 4 ] 5mmol mixes with the orga...
Embodiment 3
[0047] The difference between this embodiment and embodiment 1 is:
[0048] (1) Halogen metal salt ionic liquid [C 6 mim][CoCl 4 ] preparation: 20mmol cobalt chloride and 1-hexyl-4-methylimidazolium chloride [C 6 mim]Cl were mixed, stirred at 80°C for 8h, extracted with 15mL of dichloromethane, the resulting solution was evaporated to remove the solvent, and vacuum-dried at 80°C for 12-24h to obtain the metal salt halogen ionic liquid [C 6 mim][CoCl 4 ].
[0049] (2) Extraction of organic lignin: add 1000mL ethanol, 500mL deionized water, 8.0g sulfuric acid, and 200g wheat straw powder into a 2L stainless steel reaction kettle, react at 150°C for 6h, filter and separate, add the liquid phase After deionized water is filtered and separated, the obtained solid phase is organosoluble lignin.
[0050] (3) catalytic depolymerization of lignin: the gained halogen metal salt ionic liquid of step (1) [C 6 mim][CoCl 4 ] 10mmol mixes with the organic lignin 2.5g that step (2) ext...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com