Patents
Literature
127results about How to "Good substrate applicability" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
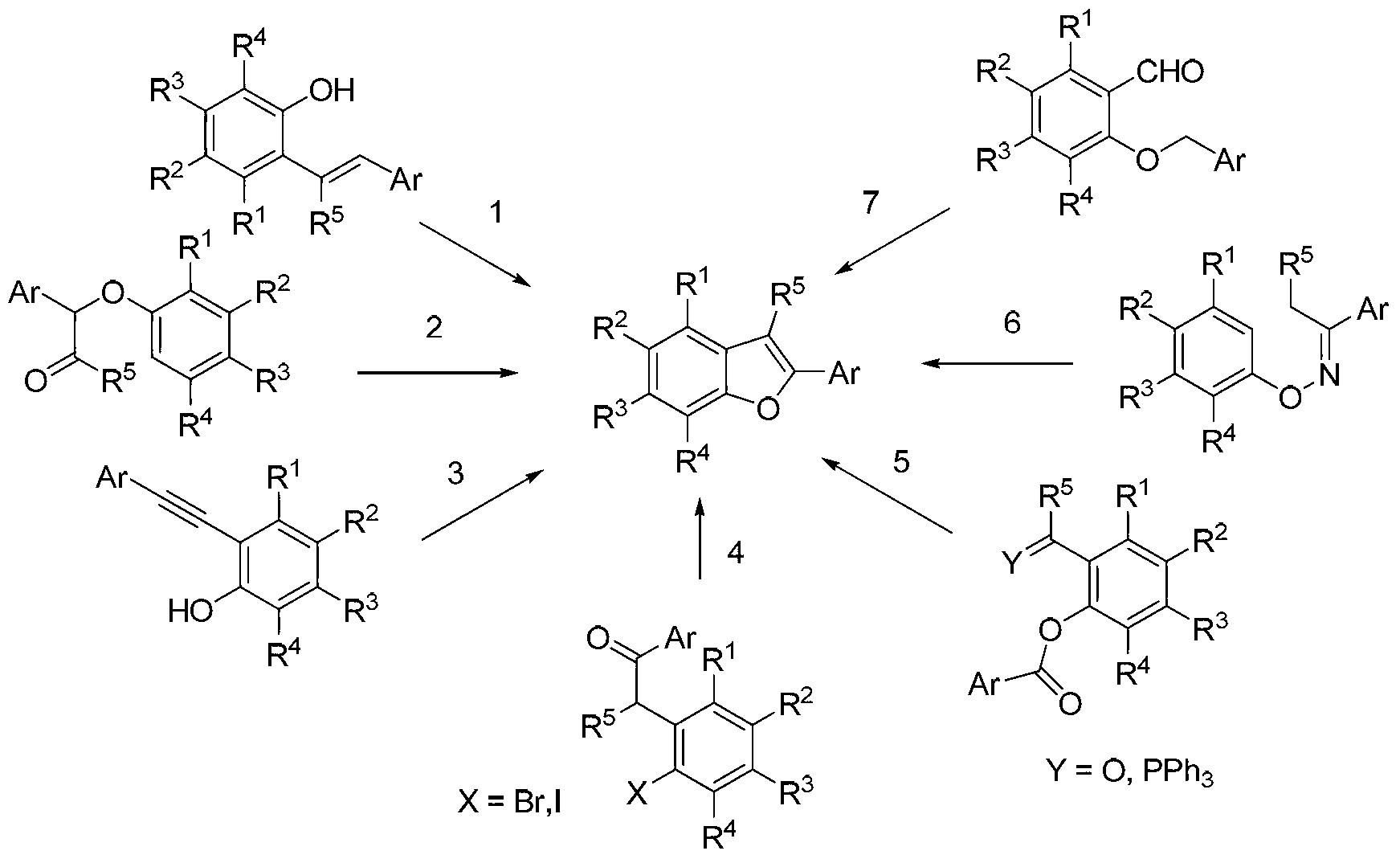
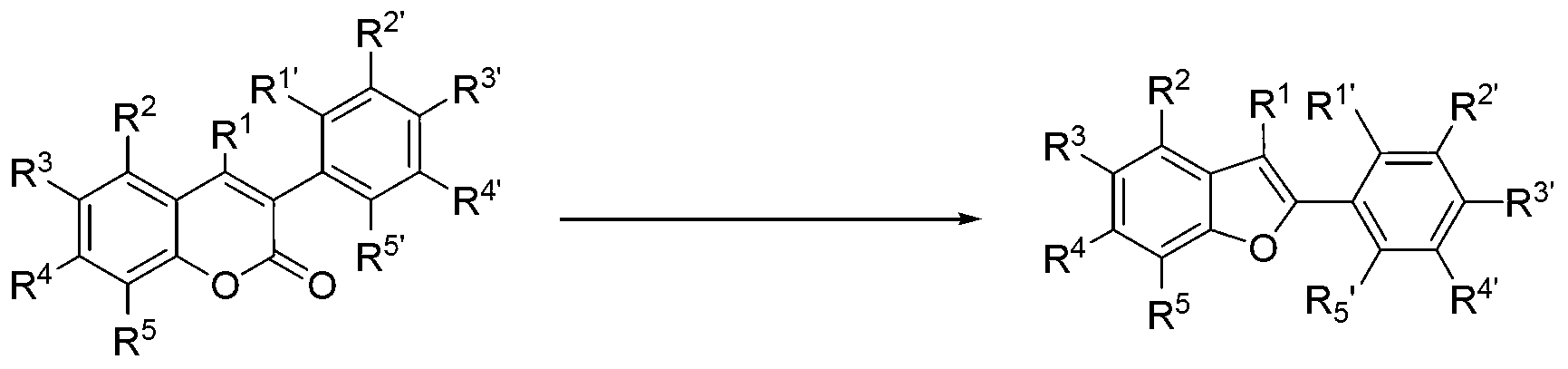
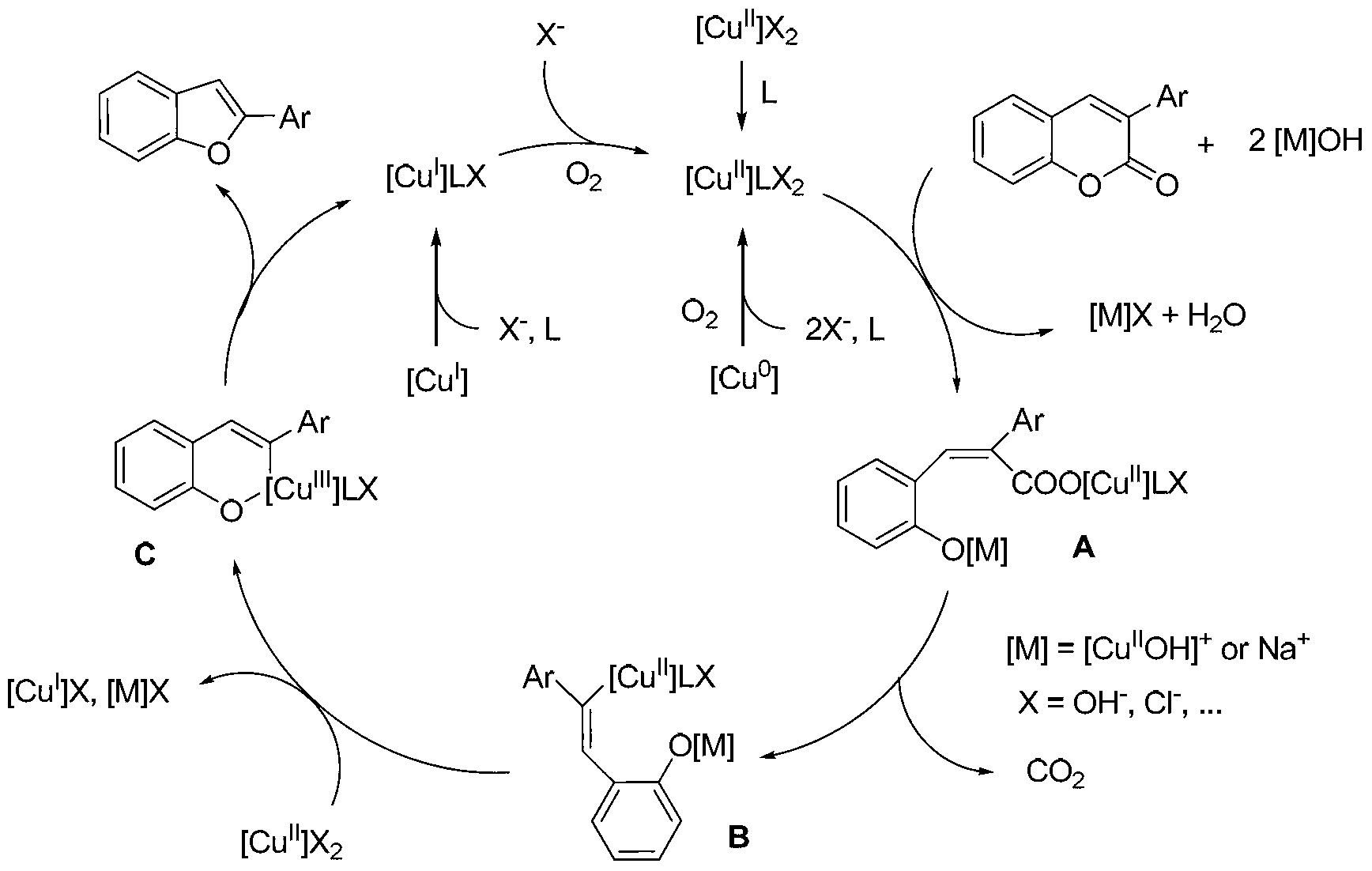
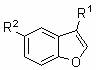
Synthesis method for 2-arylbenzofuran and derivative thereof
The invention discloses a synthesis method for 2-arylbenzofuran and a derivative thereof, aims at various degrees of technical defects existing in the conventional synthesis method for 2-arylbenzofuran and the derivative thereof, and provides a method for synthesizing 2-arylbenzofuran and the derivative thereof by taking 3-arylcumarin and a derivative of 3-arylcumarin as the raw materials. The method utilizes carbon-oxygen coupling based on metal catalysis in molecules to achieve synthesis of 2-arylbenzofuran and the derivative thereof by taking 3-arylcumarin and the derivative of 3-arylcumarin as the raw materials. A basic reaction system comprises the raw materials, an alkaline reactant, a copper catalyst, a ligand and a reaction medium, is stirred and heated to a temperature higher than 190 DEG C under an aerobic condition, maintained for 24 hours or more to prepare a reaction liquid, and is subjected to extraction and coarse product purification to prepare a final product. Optimization conditions can adopt two technical means including sectional heating and post-adding of the catalyst. The synthesis method has the advantages that the synthesis raw materials are convenient to obtain, substrate applicability is good, the synthesis technology is simple, the synthesis cost is low, and the synthesis method is particularly suitable for large-scale industrial production.
Owner:CHENGDU INST OF BIOLOGY CHINESE ACAD OF S
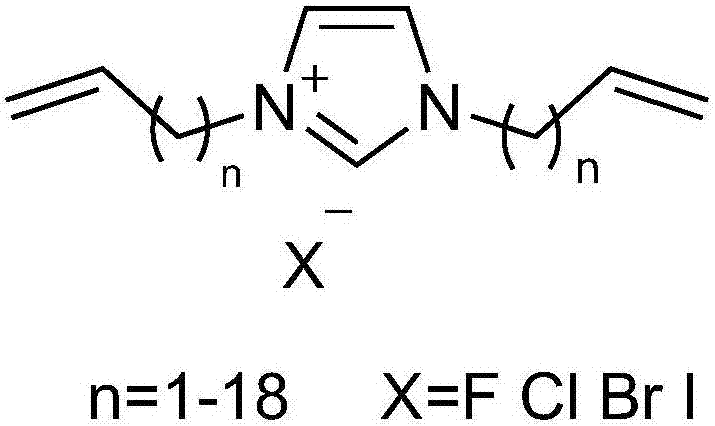
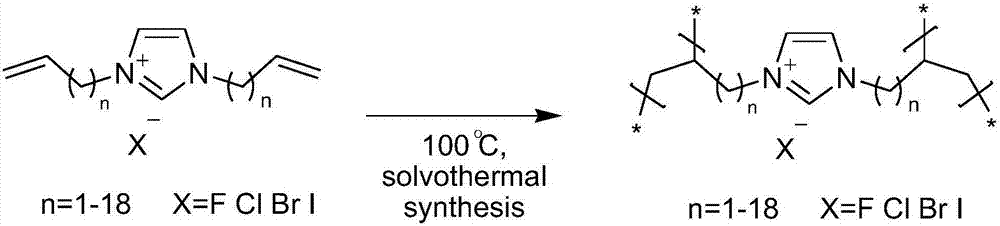
Imidazole salt organic polymer catalyst, preparation method and applications thereof
ActiveCN107537575AImprove stabilityImprove applicabilityProductsOrganic chemistryCross-linkCarbon dioxide production
The present invention relates to an imidazole salt organic polymer catalyst, a preparation method and applications thereof, wherein an alkylene functionalized imidazole salt compound is subjected to self-polymerization or is subjected mixed polymerization with a cross-linking agent to form an imidazole salt organic polymer, a Lewis acid is added or is not added to the obtained imidazole salt organic polymer, and when the Lewis acid is added, the metal ions in the acid and the N and / or P in the organic polymer are subjected to coordination so as to obtain the imidazole salt organic polymer catalyst. According to the present invention, the catalyst can be used in fixed beds, slurry beds, tank reactors, trickle beds and other reactors; with the application of the catalyst of the present invention in the cyclic carbonate production reaction of epoxy compounds and carbon dioxide, the catalyst has high activity, the generated cyclic carbonate has good selectivity, and the applicability of the substrate is strong; and the catalyst has good stability, and the separation of the catalyst from the and the reactant and the product is simple and efficient.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Organic catalysts having visible photocatalytic asymmetric hydroxylation photocatalysis performance, and preparation method and application thereof
ActiveCN107899611AEasy to separateMild reaction conditionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPhotosensitizerOrganic synthesis
The invention belongs to the technical field of visible photocatalytic asymmetric organic synthesis, and provides organic catalysts having a visible photocatalytic asymmetric hydroxylation photocatalysis performance, and a preparation method and an application thereof. The catalysts are organic catalysts having a visible photocatalytic asymmetric hydroxylation performance, constructed through combining an asymmetric organic catalyst with a visible photosensitizer through chemical bonds. The catalysts successfully realize the formation of an asymmetric C-O bond through catalytic activation of aC-H bond with molecular oxygen as an oxidizer. The invention especially relates to a most concise method for catalyzing the asymmetric alpha-hydroxylation reaction of a beta-dicarbonyl compound to prepare an alpha-chiral hydroxyl-beta-dicarbonyl compound. The method has the advantages of mild reaction conditions, good substrate applicability and environmental protection. The catalysts have the advantages of extreme easiness in separation from the substrate, stable performances, realization of keeping the catalysis effect after multi-time cycle use, and good application and development values.
Owner:DALIAN UNIV OF TECH
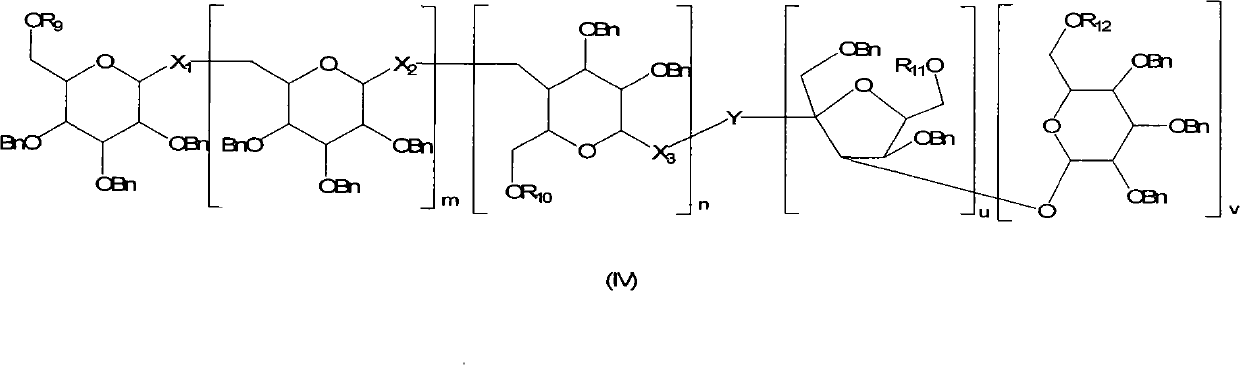
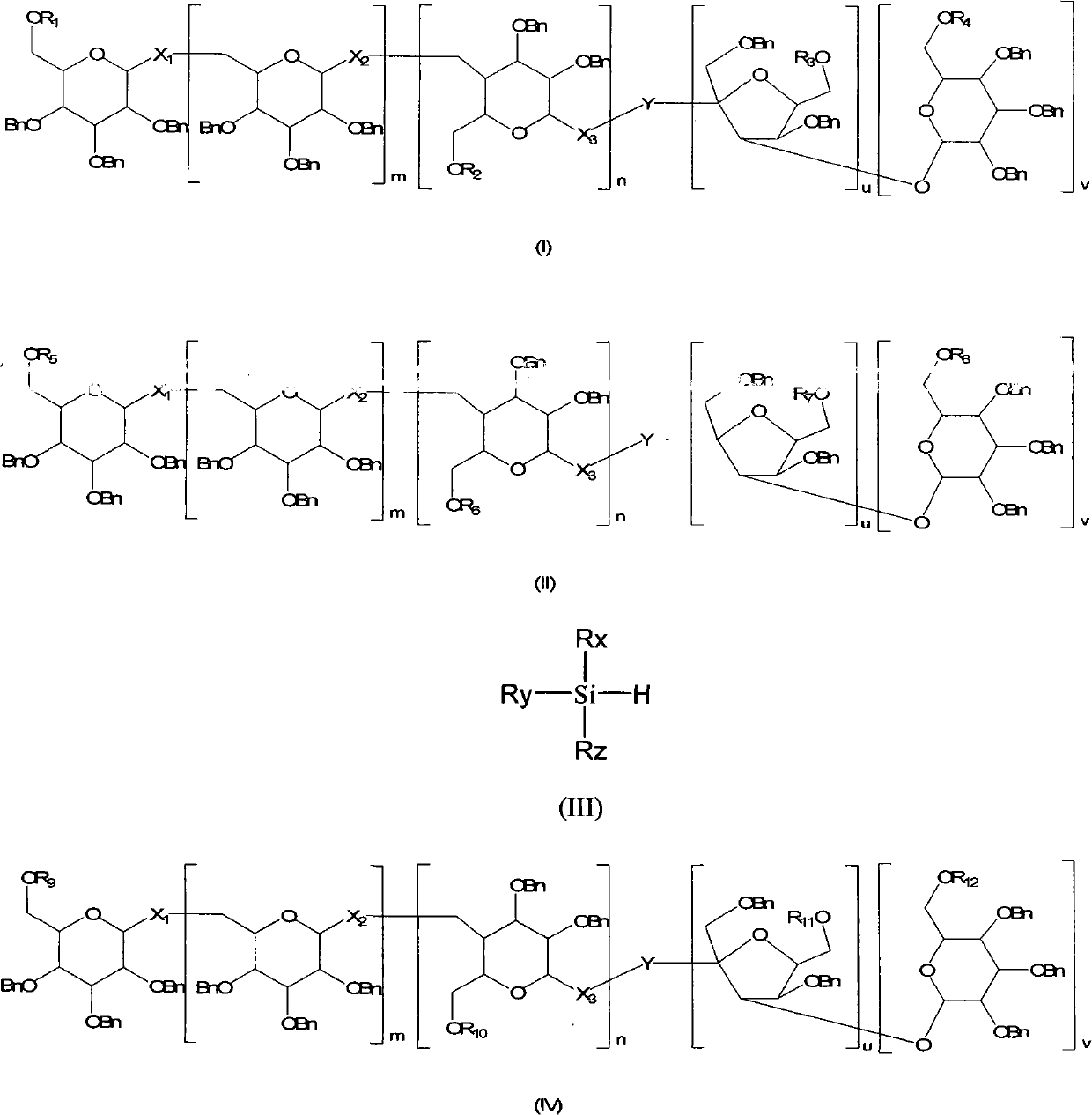
Method for regioselectively removing O-benzyl protective group of sugar
InactiveCN101775051AHigh yieldMild reaction conditionsSugar derivativesSugar derivatives preparationRegioselectivitySugar
The invention discloses a novel method for regioselectively removing O-benzyl protective group of sugar. The reaction conditions are mild and the selectivity is high. Moreover, the invention additionally provides formula (IV) intermediate compound. Definitions of substituent groups are detailed in an instruction book.
Owner:PEKING UNIV
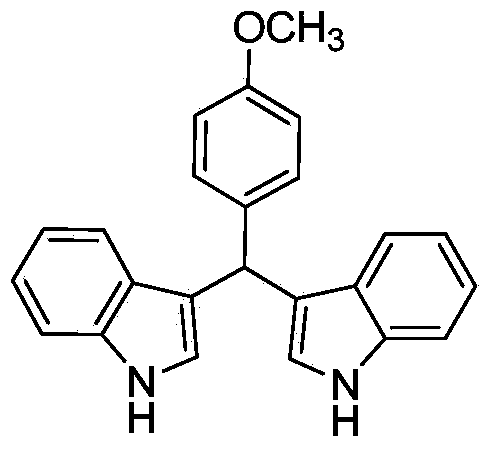
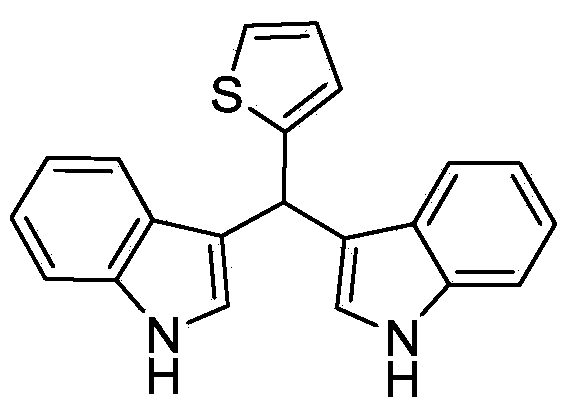
Method for preparing diindolyl methane derivatives
The invention discloses a method for preparing diindolyl methane derivatives. The method comprises the following step: with titanocene dichloride and a ligand as catalysts, reacting indole and aldehyde to obtain the diindolyl methane derivative under an alkaline condition, wherein the ligand can be 8-hydroxyquinoline, catechol and o-aminophenol and the alkali is aniline, pyridine, pyrrole or triethylamine. The method is easy to operate, the reaction is high-efficiency and mild, the catalysts are low in price, non-toxic, stable to air and water, and substrate applicability is good; therefore, the method can be widely applied to preparation of diindolyl methane derivatives.
Owner:SHAANXI NORMAL UNIV +1
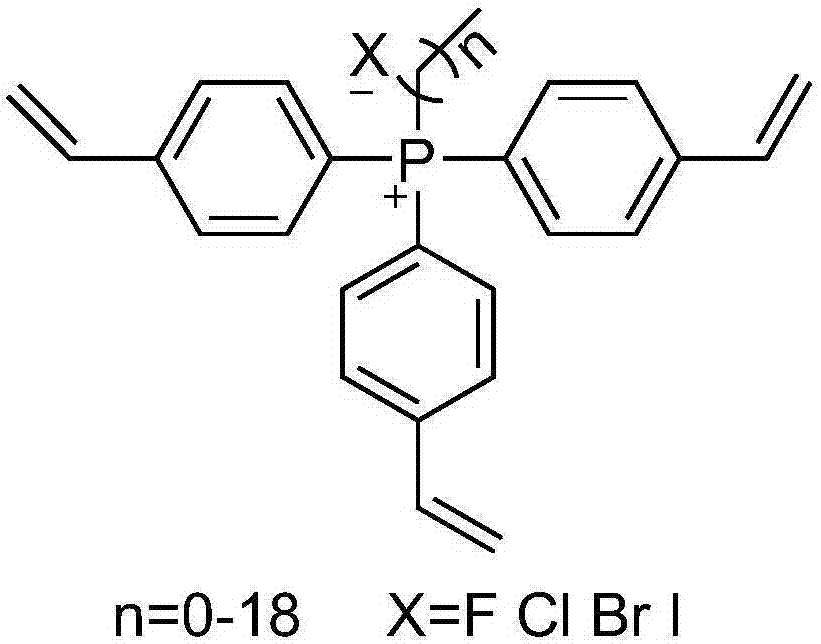
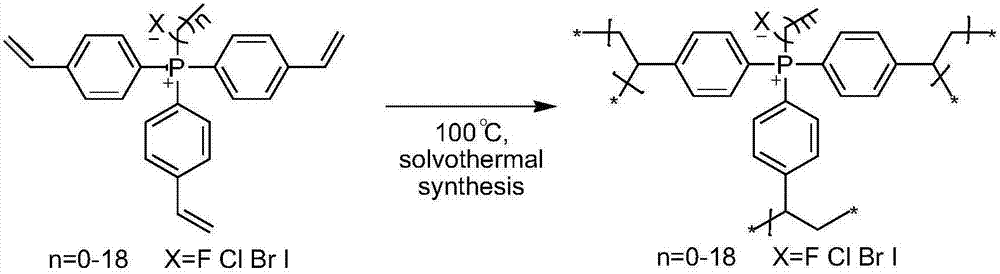
Quaternary phosphonium salt organic polymer catalyst, and preparation method and applications thereof
ActiveCN107537563AImprove stabilityImprove applicabilityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsCross-linkPhosphonium salt
The invention relates to a quaternary phosphonium salt organic polymer catalyst, a preparation method thereof, and applications of the quaternary phosphonium salt organic polymer catalyst in cyclic carbonates production. The quaternary phosphonium salt organic polymer catalyst is prepared via following steps: self-polymerization of an olefin functionalized quaternary phosphonium salt or mixing-polymerization of the olefin functionalized quaternary phosphonium salt with a cross-linking agent is carried out so as to obtain an organic polymer; a Lewis acid is added into the organic polymer, or noLewis acid is added into the organic polymer, wherein when the Lewis acid is added, coordination reaction of metal ions in the Lewis acid with N and / or P in the quaternary phosphonium salt organic polymer is realized; and an obtained product is the quaternary phosphonium salt organic polymer catalyst. The quaternary phosphonium salt organic polymer catalyst is a heterogeneous catalyst, is suitable to be used in reactors such as fixed bed reactors, slurry bed reactors, kettle-type reactors, and trickle bed reactors, possesses excellent catalytic effect in production of cyclic carbonates via addition reaction of an epoxy compound with CO2, is relatively high in catalytic activity, high in selectivity in cyclic carbonates production, high in substance applicability, and high in catalyst stability, and can be separated from reactants and products easily.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
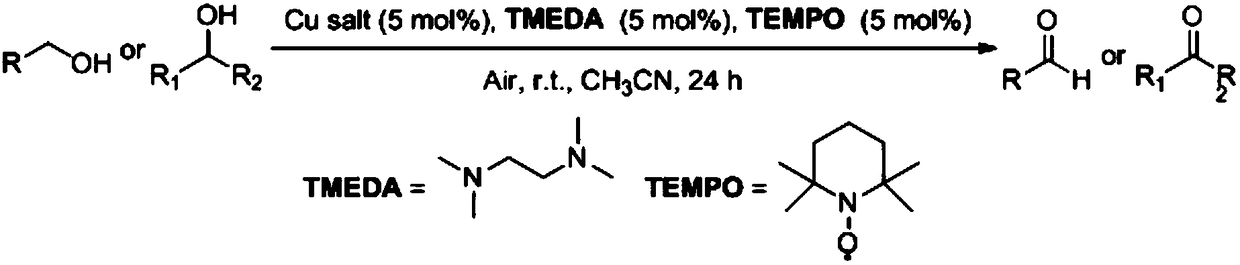
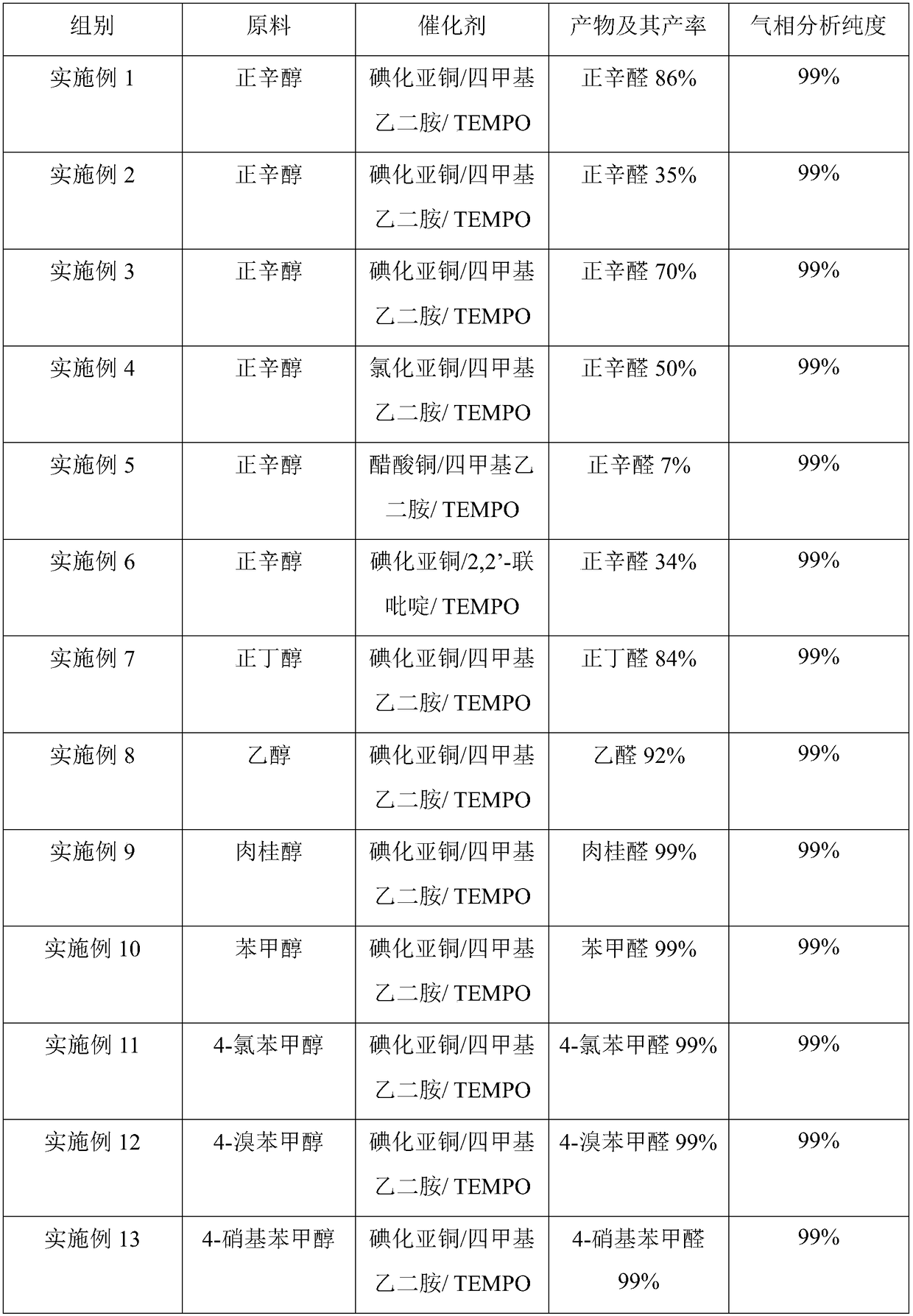
Method for preparing aldehyde or ketone by copper salt/bidentate ligand/TEMPO catalytic air oxidation alcohol
InactiveCN109096068AEasy to manufactureCheap manufacturingCarbonyl compound preparation by oxidationAlcoholKetone
The invention discloses a method for preparing aldehyde or ketone by copper salt / bidentate ligand / TEMPO catalytic air oxidation alcohol. The alcohol is used as a raw material, an organic solution is used as a solvent, and the air is used as an oxidant. The raw material alcohol is oxidized to obtain the corresponding aldehyde or ketone under the catalysis of the copper salt / bidentate ligand / TEMPO. The copper salt / bidentate ligand / TEMPO catalytic air oxidation alcohol is utilized. A technical route has the advantages of simplifying a catalytic system, being simple in operation, good in substrate applicability, high in yield, low in cost and easy to industrialize, so that the method is a very economical simple method for preparing the aldehyde or the ketone from the alcohol.
Owner:JIAXING UNIV
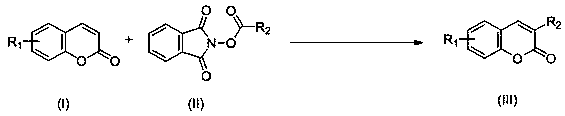
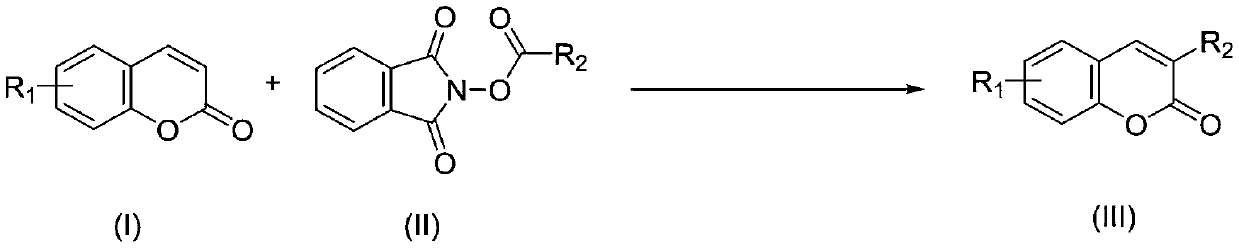
Synthesis method of C-3 alkyl substituted coumarin derivative
The invention discloses a synthesis method of a C-3 alkyl substituted coumarin derivative. The synthesis method comprises the following steps: dissolving a coumarin derivative shown as a formula (I),an N-alkyloxyphthalimide compound shown as a formula (II), a photocatalyst and a proton acid in an organic solvent; reacting under visible light irradiation at a temperature of 20 to 60 DEG C for 3 to36 hours; after the reaction is finished, performing post-treatment on a reaction system to obtain a target product of the C-3 alkyl substituted coumarin derivative shown as a formula (III), whereinthe reaction formulas are shown in the description; in the formulas (I) and the formula (III), the substituent R1 is H, methyl, methoxy, fluorine, chlorine or bromine; in the formula (II) and the formula (III), the substituent R2 is linear alkyl or naphthenic alkyl of with 1 to 8 carbon atoms. The synthesis method realizes the C-3alkylation of coumarin with light as reaction energy, makes the reaction safer, more environmentally friendly and cheaper, expands the application range of the reaction substrate, and enriches the synthesis method of a C-3 substituted coumarin compound.
Owner:ZHEJIANG UNIV OF TECH
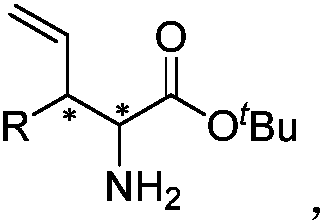
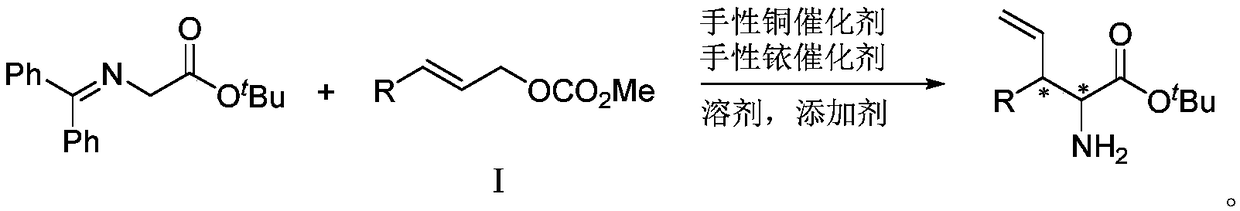
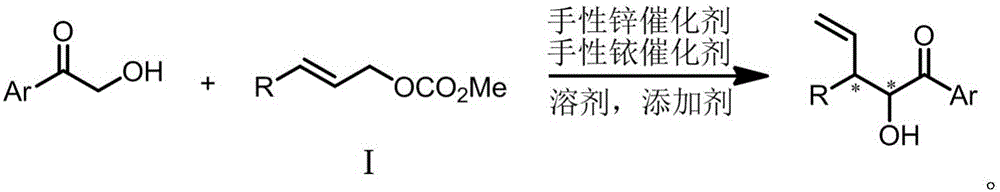
Chiral 3-substituted 3-vinyl-2-amino propionate and preparation method thereof
InactiveCN109438269AStereo-divergent synthesisGood substrate applicabilityOrganic compound preparationAmino-carboxyl compound preparationPropionateNatural product
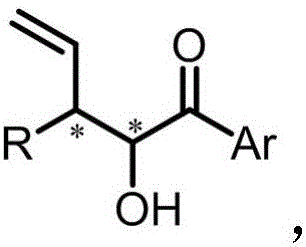
The invention provides chiral 3-substituted 3-vinyl-2-amino propionate. The structural formula of the chiral 3-substituted 3-vinyl-2-amino propionate is as shown in specification. The invention further provides a method for synthesizing the 3-substituted 3-vinyl-2-amino propionate, and a reaction route of the method is as shown in specification. The chiral 3-substituted 3-vinyl-2-amino propionatehas the advantages of high substrate applicability, mild reaction conditions, simple operation, low synthesis cost and the like, and stereoscopic divergent synthesis of the 3-substituted 3-vinyl-2-amino propionate with continuous chiral centers can be implemented by only one-step reaction. The product can be directly applied to selective synthesis of an important chiral amino acid fragment of a marine natural product Hal ipeptin A with high anti-inflammatory activity, and thus, the original process is greatly shortened.
Owner:SHANGHAI UNIV OF MEDICINE & HEALTH SCI
Method for preparing aldehyde ketone by efficiently catalyzing molecular oxygen to oxidize alcohol by taking hydrotalcite-like material as catalyst
InactiveCN107176898AGood substrate applicabilityEfficient CatalysisOrganic compound preparationCarbonyl group formation/introductionAlcoholHydrotalcite
The invention belongs to the technical field of liquid-phase catalytic oxidation and provides a method for preparing aldehyde ketone by efficiently catalyzing molecular oxygen to oxidize alcohol by taking a hydrotalcite-like material as a catalyst. The catalyst can be expressed as A<n->-NixM-LDHs (A<n->=OH<->, CO3<2->, CH3COO<-> and PO4<3->; M is Ga or In; the ratio of Ni to M is (2-4) to 1). The method is characterized by preparing aldoketones compounds by carrying out aerobic oxidation reaction on alcohol under mild condition without adding additives in the presence of the catalyst. The hydrotalcite-like material can be synthesized in quantity and can be recycled; the method has the advantages of high selectivity and yield of aldehyde ketone, mild reaction condition, low cost and easiness in realizing industrialization.
Owner:CHANGZHOU UNIV
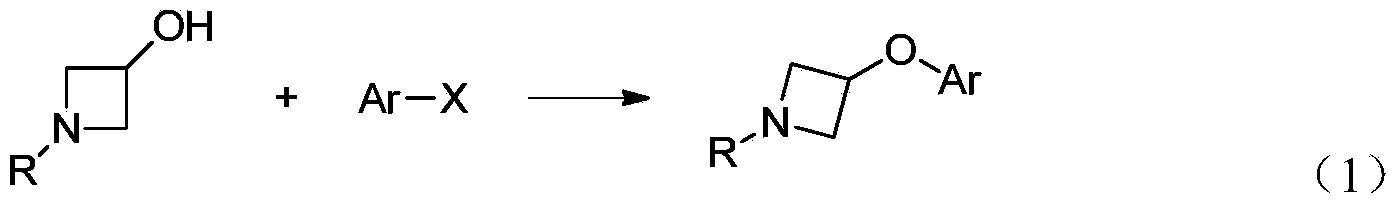
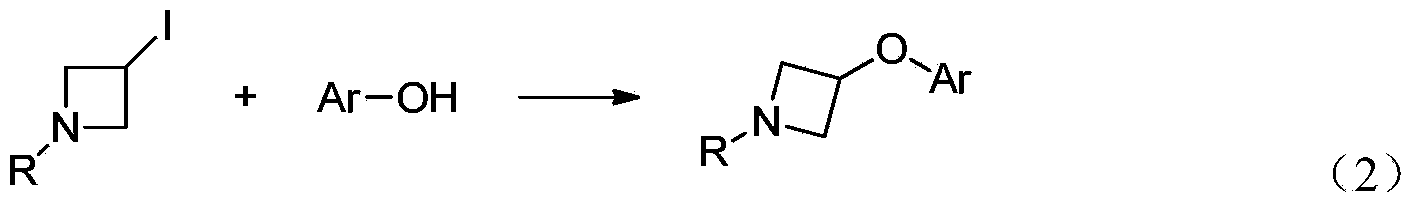
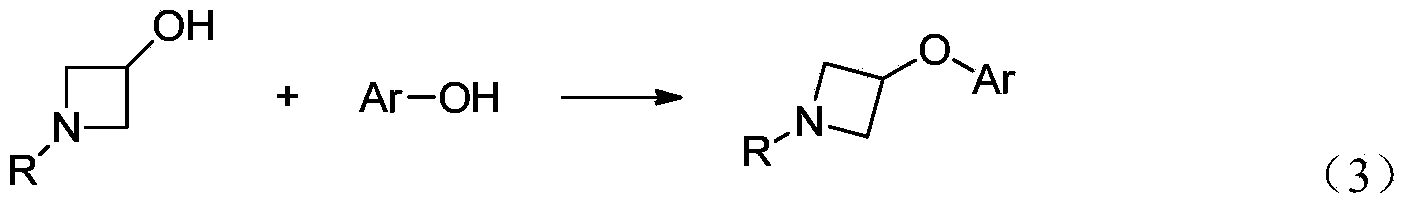
Preparation method for N-tert-butyloxycarboryl-azetidine aromatic ether/aromatic heterocyclic ether compounds
ActiveCN104140387AWide applicabilityImprove stabilityOrganic chemistryTert-Butyloxycarbonyl protecting groupOrganic synthesis
The invention discloses a preparation method for N-tert-butyloxycarbonyl-azetidine aromatic ether / aromatic heterocyclic ether compounds, and belongs to the technical field of organic synthesis. The method is as follows: by taking 1-tert-butyloxycarboryl-3-iodoazetidine and arylboronic acid or nitrogen-containing heterocyclic boric acid as materials, producing the N-tert-butyloxycarboryl-azetidine aromatic ether / aromatic heterocyclic ether compound by virtue of copper-catalyzed carbon-oxygen cross-coupling reaction. The reaction conditions are gentle, the substrate applicability is good, the reaction specificity is strong, and a medium to high yield can be obtained. A catalyst system for the reaction has good stability, efficient catalytic activity and extensive applicability, and can effectively avoid damages of strong-basicity reaction conditions on a certain functional groups; moreover, the materials are cheap and easy to obtain, so that the cost is low.
Owner:TYK MEDICINES ZHENGZHOU INC +1
Method for synthesizing alpha, beta-unsaturated amide compound through visible light catalysis
ActiveCN110818584AEfficient introductionQuick introductionOrganic compound preparationCarboxylic acid amides preparationOrganic synthesisVisible light radiation
The invention discloses a method for synthesizing an alpha,beta-unsaturated amide compound through visible light catalysis, and belongs to the technical field of organic synthesis. The method comprises the following synthesis steps: subjecting a phenylethylene compound and a formamide compound to a reaction under the conditions of a nitrogen atmosphere and a temperature and under the actions of visible light radiation and synergistic effects of an oxidant and a photocatalyst, and carrying out extracting, drying, concentrating and purifying so as to obtain the alpha,beta-unsaturated amide compound. The method provided by the invention has the advantages of simple and easily-available raw materials, stable chemical properties, mild reaction conditions, simple operation, strong substrate applicability and the like, can be widely applied to drug synthesis and organic molecule synthesis, and has wide industrial application prospects.
Owner:MINNAN NORMAL UNIV
Transition metal natural polymer hybridization catalyst as well as preparation method and application thereof
ActiveCN108554451ALow costEasy to prepareOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsKetoneTransfer hydrogenation
The invention belongs to the technical field of preparation of hybridization catalysts and in particular relates to a transition metal natural polymer hybridization catalyst as well as a preparation method and application thereof. According to the method provided by the invention, lignin and a group VB transition metal salt solution are subjected to hydrothermal reaction; the preparation method issimple and the transition metal natural polymer hybridization catalyst prepared by the method has good stability and can be used for efficiently catalyzing transfer hydrogenation reaction of aldehyde, ketone or levulinate and alcohol organic matters; the conversion rate of preparing alcohols from aldehydes reaches 84 percent to 99 percent and the selectivity of the alcohols reaches 80 to 100 percent. Furthermore, in an application process of the catalyst, the applicability of a substrate is good and the catalyst is easy to separate after reaction. The lignin is used as a raw material so thatthe catalyst is safe and low in cost and can be used for converting the lignin with low value into high value; the catalyst is green and environmentally friendly and can be circularly regenerated, andindustrialization is easy to realize.
Owner:SOUTH CHINA UNIV OF TECH
3-substituted 3-vinyl-2-hydroxy-1-aryl acetone and synthetic method thereof
ActiveCN106316813AGood substrate applicabilityMild reaction conditionsOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsDivergent synthesisBroad spectrum
The invention provides a method for synthesizing 3-substituted 3-vinyl-2-hydroxy-1-aryl acetone, and the reaction route is shown as follows: (please see the formula in the description), wherein Ar is phenyl, thienyl or substituted phenyl; R is methyl, phenyl, furyl or substituted phenyl. Compared with the existing allyl synthesis of alpha position of hydroxyketone, the synthetic method has the advantages of having hydroxyl group, requiring no protection, and being good in the substrate applicability, mild in the reaction conditions, simple in operation and low in the synthetic cost, and stereo divergent synthesis of 3-substituted 3-vinyl-2-hydroxy-1-aryl acetone with continuous chiral centers can be achieved only through one-step reaction, namely, any one of the four spatial configurations of the product is synthesized according to the need. The product can be directly applied to the selectivity synthesis of the broad-spectrum inhibitor hinokiresinol medicine, and reduce the original process.
Owner:SHANGHAI JIAO TONG UNIV
Method for synthesizing end alkynyl by utilizing 1,1-two bromination vinyl compound
InactiveCN102001902AEasy to operateImprove economyAmino preparation from aminesOrganic chemistry methodsBiochemical engineeringOrganic synthesis
The invention belongs to the technical field of organic synthetic intermediates and particularly relates to a method for synthesizing end alkynyl by utilizing 1,1-two bromination vinyl compound. The end alkynyl compound is synthesized by using the 1,1-two bromination vinyl compound as raw materials under the alkali condition. The end alkynyl is an important organic synthetic raw material and an intermediate, and can be widely applied to the synthesis of medicines, agricultural medicines, materials and functional molecules. The invention has the advantages of simple operation, mild condition, high yield and the like, thereby having wide application prospect.
Owner:TONGJI UNIV
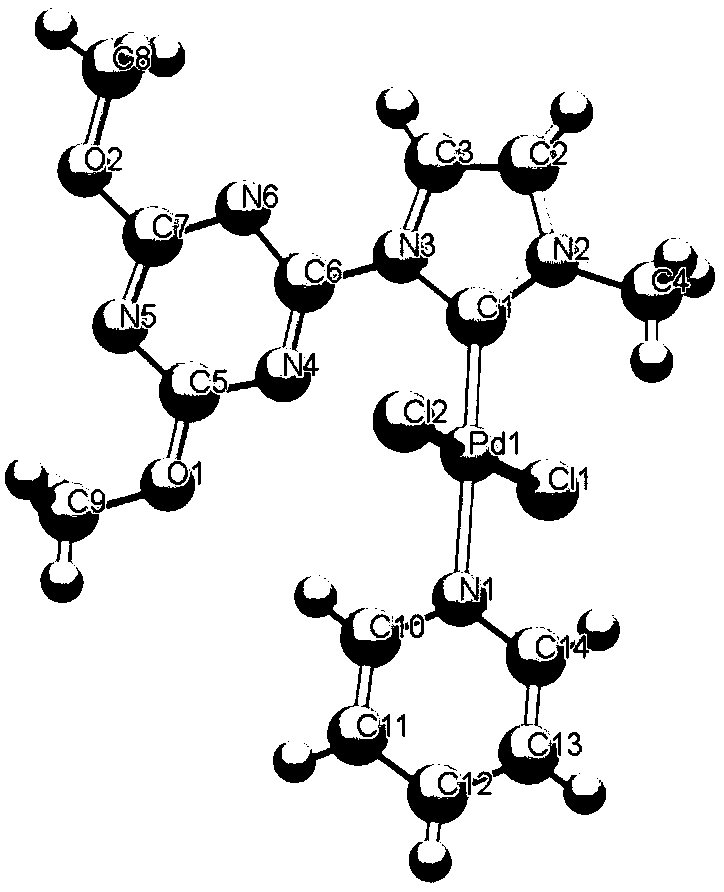
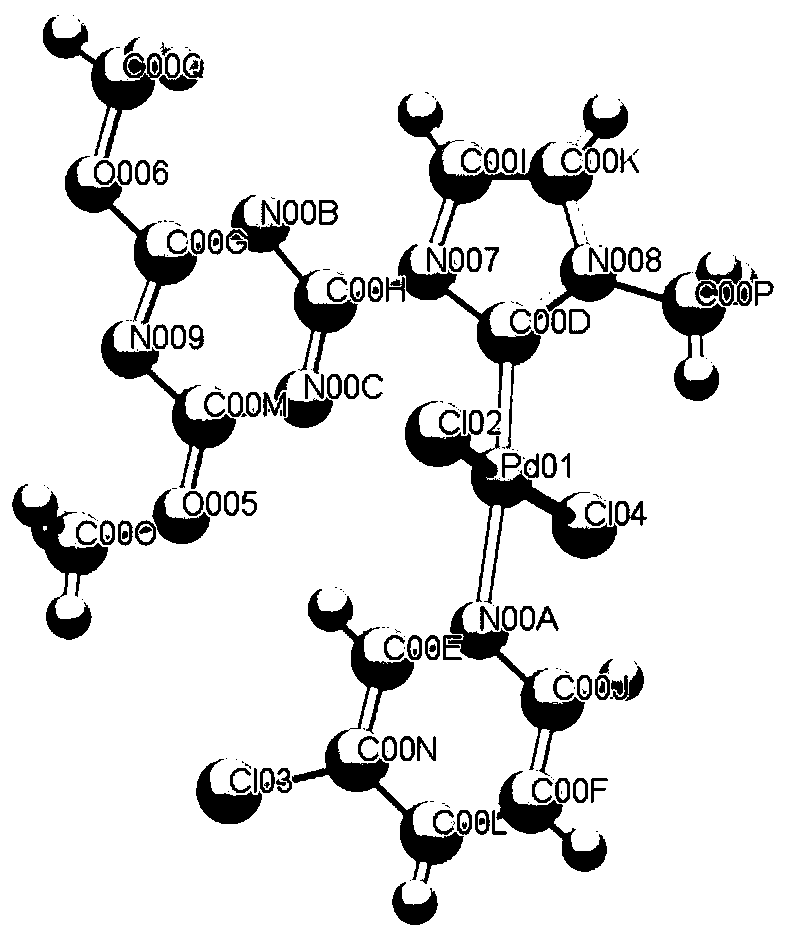
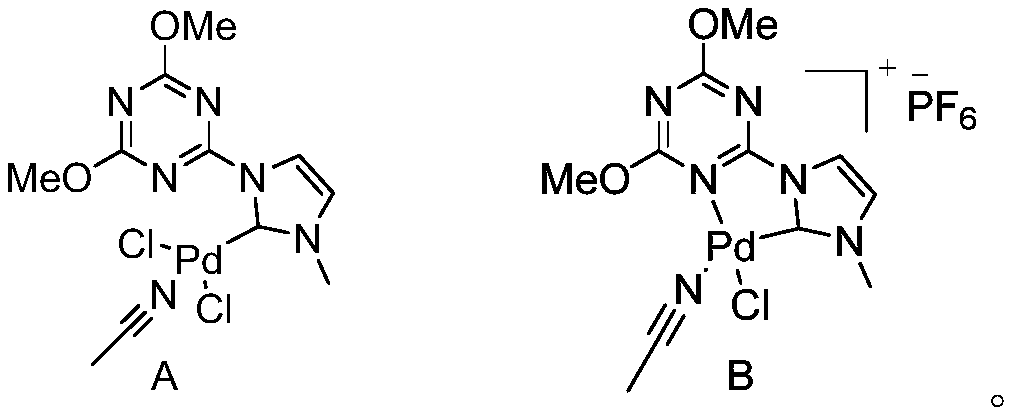
N-heterocyclic carbene palladium complex crystal, synthetic method thereof, and application thereof in preparation of amide compounds
ActiveCN110483582AIncrease electron densityHigh electron affinityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSynthesis methodsCarbene
The invention discloses an N-heterocyclic carbene palladium complex crystal, a synthetic method thereof and application thereof in preparation of amide compounds. The structural formula of the complexcrystal is shown in the specification, or the complex crystal is prepared from an N-heterocyclic carbene ligand, Pd(CH3CN)2Cl2 and Ag2O in acetonitrile serving as a solvent. The N-heterocyclic carbene palladium complex crystal is relatively stable, wherein the raw materials used in the synthesis method are cheap and easy to obtain, so that the synthetic method is simple, convenient and easy to implement is simple in post-treamtent and is high in yield; and the N-heterocyclic carbene palladium complex crystal is used for catalyzing carbonylation carbon-nitrogen bond coupling reaction to prepare amide compounds and is high in catalytic activity, easy to operate and high in atom economy.
Owner:SHAANXI NORMAL UNIV
Synthesis method of alpha-F-beta-OH-carbonyl compound
ActiveCN109734571AReact greenLow costOrganic compound preparationCarbonyl compound preparationSynthesis methodsSolvent
The invention discloses a synthesis method of an alpha-F-beta-OH-carbonyl compound. The synthesis method comprises the following steps: dissolving an unsaturated ketone compound shown in formula (I) and an N-F reagent in a reaction solvent, reacting for 5 to 25 hours at the temperature of 30 to 100 DEG C, after the reaction is ended, performing the after-treatment for a reaction solution to obtainan alpha-F-beta-OH-carbonyl compound as shown in formula (II), wherein the reaction formula is shown as follows: (shown in the description) In formula (I) and (II), substituent groups R1 and R2 are respectively independently selected from phenyl, substituted phenyl, thienyl or furyl; and the substituent group of the substituted phenyl is at least one of C1 to C3 alkyl, C1 to C3 alkoxy, nitro, trifluoromethyl, fluorine, chlorine and bromine. The N-F reagent is used as a fluorine source and Lewis acid, no additional catalyst is used, so that the reaction is more environment-friendly, and the cost is lower; and the method for synthesizing the alpha-F-beta-OH-carbonyl compound provided in the invention has the advantages of easy availability of raw materials, mild reaction conditions, no needof other catalysts, good reaction selectivity, simplicity in operation and the like.
Owner:ZHEJIANG UNIV OF TECH
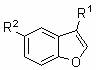
Method for synthesizing alpha-benzyl benzofuran compound
PendingCN112645909AAchieving a hydroheteroarylation reactionEasy to synthesizeSilicon organic compoundsOrganic-compounds/hydrides/coordination-complexes catalystsArylPtru catalyst
The invention discloses a method for synthesizing an alpha-benzyl benzofuran compound, which comprises the following step of: reacting a benzofuran compound and an aryl ethylene compound serving as raw materials in a solvent in the presence of a catalyst and organic alkali in an inert gas atmosphere to obtain the alpha-benzyl benzofuran compound. Compared with the existing method, the method disclosed by the invention has the advantages that the direct use of an air-sensitive zero-valent nickel complex as a catalyst is avoided, and the applicability of the substrate is obviously expanded; provided is a novel method for producing the alpha-benzyl benzofuran compound by a hydroheteroarylation reaction of an arylethene compound and a benzofuran compound, said method being achieved by an air-stable divalent nickel-based catalyst.
Owner:SUZHOU UNIV
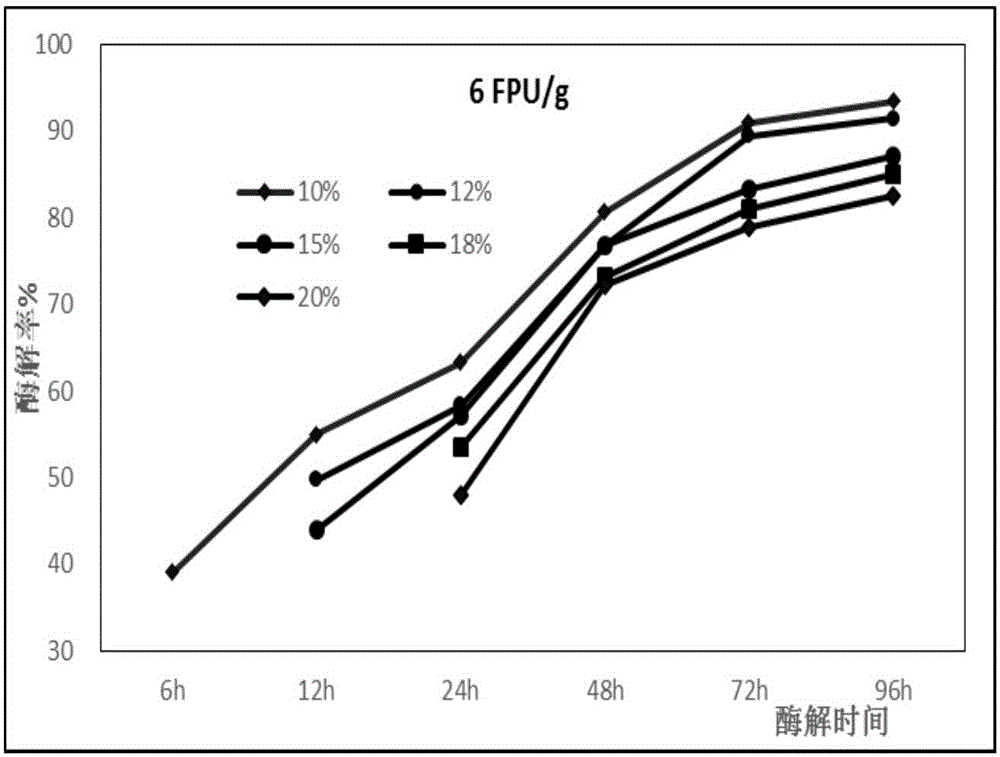
Method for hydrolyzing thick mash of agricultural and forest biomass raw material to produce glycose
The invention relates to a method for hydrolyzing thick mash of agricultural and forest biomass raw material to produce glycose. The method is characterized in that fiber biomass is used as the raw material, and a normal-pressure instantaneous glycerin organic phase boiling pretreatment method is adopted, so that while more than 90% of cellulose in raw material is maintained, more than 70% of lignin and less than 20% of cellulose can be selectively removed; a large amount of glycerol glycoside is produced in the collected glycerin treatment liquid, but the furfural type fermenting inhibiting matter is basically not produced, and the glycerin can be directly recycled for 7 to 11 times; when the crude cellulose of glycerin has 10 to 40% of matrix concentration and 2 to 15FPU.g<-1> matrix enzyme carrying amount, the 72hr enzymatic hydrolysis ratio can reach 70% or more. The method for hydrolyzing the thick mash of the agricultural and forest biomass raw material to produce the glycose has the characteristics that the simplicity and economy are realized, the efficiency is high, the environment-friendly effect is realized, and the simultaneous commercialized application prospect of biodiesel and cellulosic ethanol is realized.
Owner:JIANGNAN UNIV
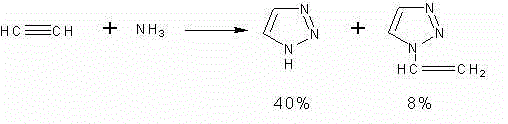
Synthetic method for N-vinyl-triazole compound
InactiveCN103333127AEasy to operateImprove economyOrganic chemistryOrganic synthesisCarvacryl acetate
The invention provides a synthetic method for an N-vinyl-triazole compound, particularly relates to the fact that the N-vinyl-triazole compound is synthesized by 1,2,3-triazole, 1, 2, 4-triazole, 1, 3, 4-triazole compounds and vinyl acetate under the reaction condition of trifluoroacetic acid / acetic acid as a catalyst, and belongs to the technical field of organic synthesis. A triazole compound, namely a five-membered ring containing three nitrogen and derivant of the five-membered ring have wide biological activity, and a coordination compound formed by the triazole compound and metal has many remarkable application prospects on function materials, organic synthesis, medicine, medical science and the like. Based on the view point of the high molecule structural design, the coupled reaction of substituent of vinyl and various functional triazole groups is realized to prepare novel allyl monomers used for polymerization reaction, and the allyl monomers can be used for preparing functional polymer materials with different molecular weight in a homopolymerisation and copolymerization manner.
Owner:孟祥英
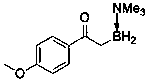
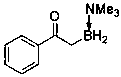
Method for synthesizing alpha-borocarbonyl compound through B-H bond insertion reaction with iridium as catalyst and sulfur ylide as Carbene precursor
InactiveCN110590821AGood substrate applicabilityBroad application prospectsGroup 3/13 element organic compoundsCarbeneChemistry
The invention relates to a method for efficiently synthesizing an alpha-borocarbonyl compound through B-H insertion reaction with iridium as a catalyst and sulfur ylide as a Carbene precursor and lewis base borane adduct. Compared with other methods, the method has the advantages that the raw materials are stable and easy to obtain, the steps are simple, the conditions are mild, the method can effectively prepare the important molecular entity, namely the alpha-borocarbonyl compound, in organic synthesis, and the method has wide application prospects.
Owner:SICHUAN UNIV
Method for preparing alpha, beta-acetylenic ketone compounds through carbon monoxide-releasing molecular carbonylation carbon-carbon bond coupling
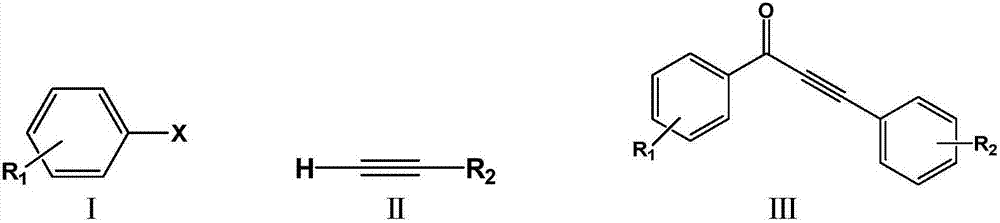
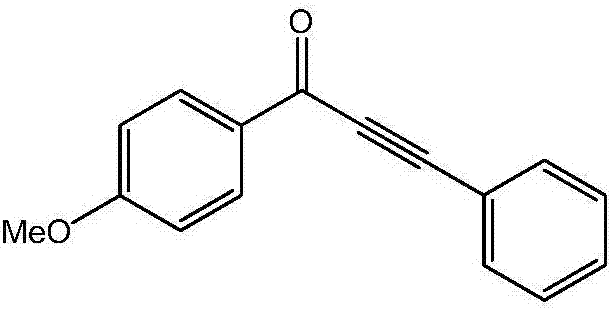
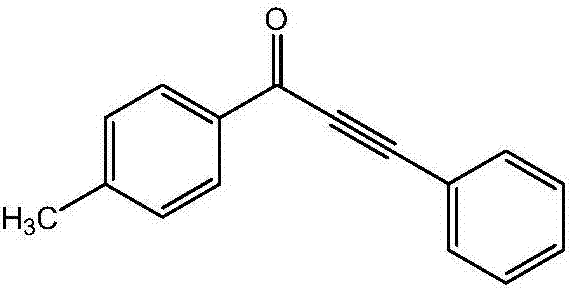
ActiveCN106866390AReduce usageEasy to operateOrganic compound preparationCarbonyl compound preparationCarbon–carbon bondKetone
The invention discloses a method for preparing alpha, beta-acetylenic ketone compounds through carbon monoxide-releasing molecular carbonylation carbon-carbon bond coupling. According to the method, CO gas is not used, and the method comprises the following steps: by taking palladium chloride as a catalyst, taking 4,5-bis (diphenylphosphino)-9,9-dimethyl xanthenes as a ligand, taking N, N-dimethyl formamide as a solvent, taking pentacarbonyl iron as a CO supply source of a carbon monoxide-releasing molecule, coupling aryl halides and terminal alkyne under alkaline conditions, thereby obtaining the alpha, beta-acetylenic ketone compounds. According to the method disclosed by the invention, usage of high-pressure poisonous gas carbon monoxide is avoided, and the method is simple and safe in experimental operation, mild in conditions and high in substrate applicability and can be widely applied to preparing the alpha, beta-acetylenic ketone compounds.
Owner:SHAANXI NORMAL UNIV
Method for preparing N-heterocyclic aromatic hydrocarbon derivatives by dehalogenation and alkylation
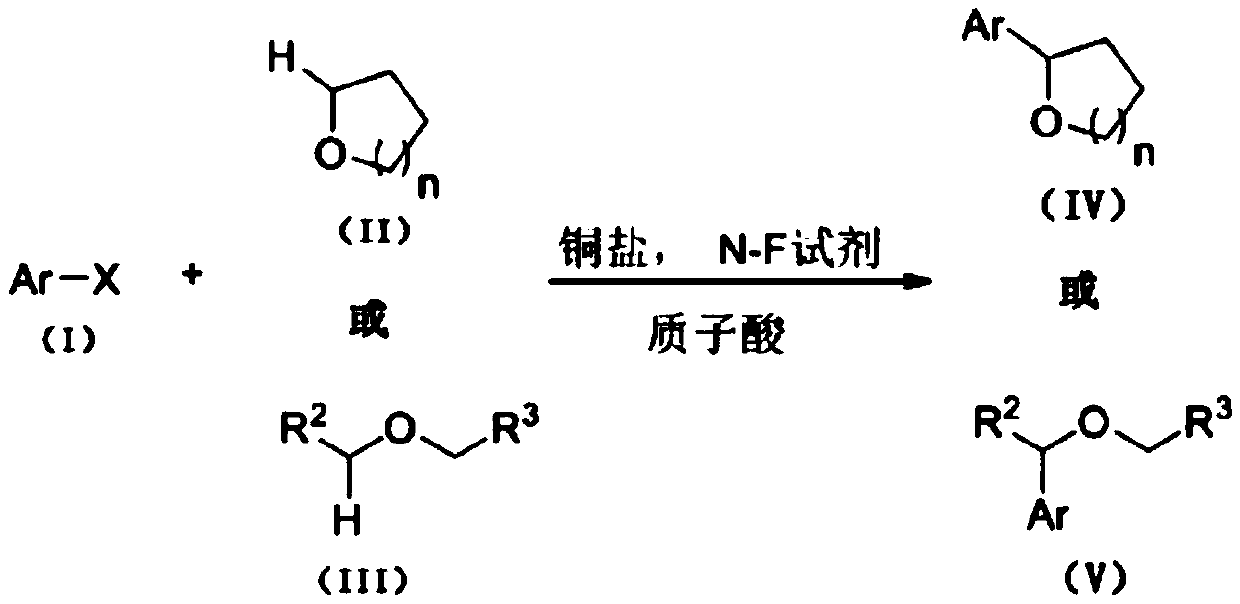
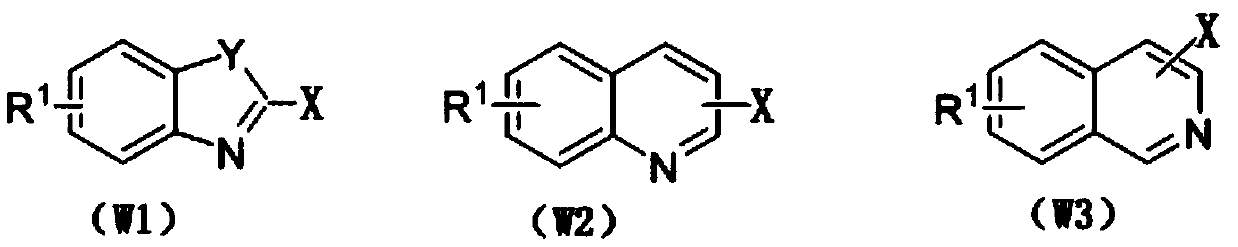
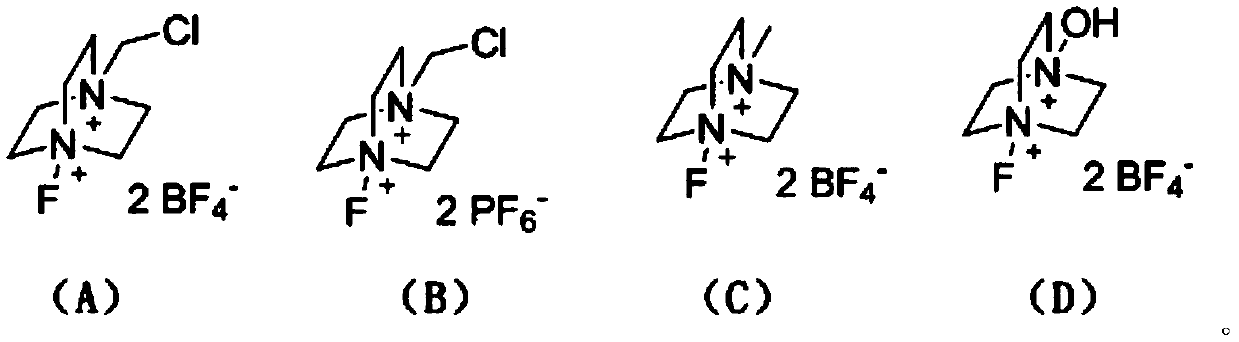
The invention discloses a method for preparing N-heterocyclic aromatic hydrocarbon derivatives by dehalogenation and alkylation. The method includes: dissolving halogenated N-heterocyclic aromatic hydrocarbon compounds, ether compounds, copper salts, an N-F reagent and protonic acid into an organic solvent, stirring in oil bath, performing reaction for 3-20h at a temperature of 20-60 DEG C, and after reaction is finished, treating reaction liquid to obtain the N-heterocyclic aromatic hydrocarbon derivatives. The method has advantages that dehalogenation and alkylation of the N-heterocyclic aromatic hydrocarbon compounds are realized under catalysis of the copper salts, high regioselectivity in reaction is achieved, a reaction substrate application range is expanded, and selective dehalogenation and alkylation of halogenated heterocyclic aromatic hydrocarbon compounds such as halogenated quinoline derivatives, halogenated isoquinoline derivatives, halogenated benzoxazole derivatives andhalogenated benzothiazole derivatives can be realized.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of substituted urea compound
ActiveCN112920140AWide variety of sourcesAtom utilization is highOrganic chemistryPtru catalystNitrogenous heterocyclic compound
The invention provides a preparation method of a substituted urea compound. The preparation method comprises the following steps: taking a nitrogen-containing heterocyclic compound and tetrahalomethane as raw materials, carrying out illumination catalytic reaction in the presence of a catalyst and a solvent to prepare the substituted urea compound and a halogen simple substance; the preparation method is wide in raw material source, phosgene, triphosgene and the like which are high in toxicity are prevented from being adopted as raw materials, the influence of the preparation process on the environment is reduced, and the atom utilization rate of the reaction is increased; the reaction conditions are mild, the operation is simple and convenient, the environmental pollution is reduced, and the reaction cost is reduced; the product yield is high, the halogen simple substance is co-produced, the additional value is high, a large amount of waste is prevented from being generated, and the method has high atom economy and environment friendliness and is beneficial to application and popularization.
Owner:CENT SOUTH UNIV +1
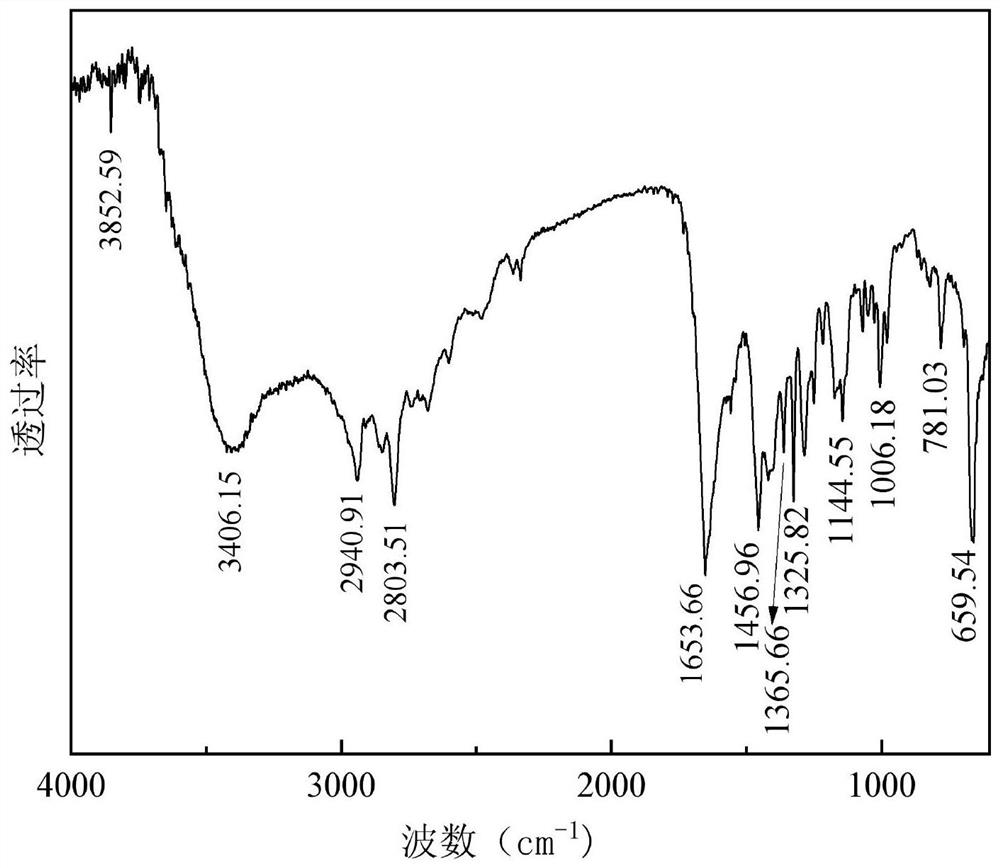
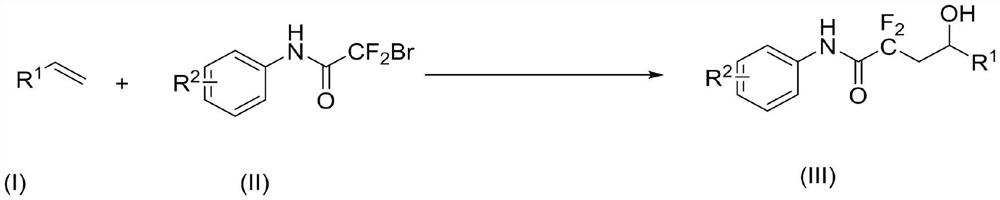
Synthesis method of alpha, alpha-difluoro-gamma-hydroxyacetamide derivative
ActiveCN112574056AReact SafeReact greenOrganic compound preparationCarboxylic acid amides preparationOrganic baseAniline
The invention discloses a synthesis method of an alpha, alpha difluoro gamma hydroxyacetamide derivative, which comprises the following steps: dissolving a bromo difluoro acetanilide compound shown ina formula (II), a photocatalyst and an organic alkali in an olefin derivative shown in a formula (I), reacting at room temperature for 5-24 hours under the irradiation of visible light, and after thereaction is finished, filtering to obtain the alpha, alpha difluoro gamma hydroxyacetamide derivative. A reaction system is subjected to post-treatment to obtain the alpha, alpha difluoro gamma hydroxyacetamide derivative shown in the formula (III), and the reaction formula is shown in the specification, in the formula (I) and the formula (III), a substituent group R1 is C2-C11 linear alkyl, arylor substituted aryl; in the formula (II) and the formula (III), the substituent R2 is H, methyl, methoxy, fluorine, chlorine, bromine or aromatic heterocycle. According to the invention, light is used as a reaction energy source to realize hydroxylation of olefin acetamide, so that the reaction is safer and greener, the cost is lower, the substrate application range of the reaction is expanded, and a simpler and more convenient method for preparing the alpha, alpha-difluoro gamma-hydroxyacetamide compound is provided.
Owner:ZHEJIANG UNIV OF TECH
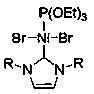
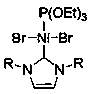
Application of N-heterocyclic carbene-based mixed nickel (II) complex in reaction of synthesizing 2-linear alkylbenzothiazole compound
ActiveCN111420709AAchieving a hydroheteroarylation reactionMild reaction conditionsOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystCarbene
The invention discloses an application of an N-heterocyclic carbinyl mixed nickel (II) complex in a reaction for synthesizing a 2-linear alkyl benzothiazole compound and a method for synthesizing the2-linear alkyl benzothiazole compound. The mixed nickel (II) complex containing Ni[P(OEt)3](RNCHCHNR)C]Br2[R is 2, 4, 6, 7, 8-tetramethyl-4-piperidinecarboxylic acid] is used as a catalyst, and the 2-linear alkyl benzothiazole compound is synthesized through a hydrogen heteroarylation reaction of aliphatic alpha-olefin and a benzothiazole compound in the presence of magnesium. Compared with the prior art, the invention avoids the use of expensive monovalent rhodium complexes, and provides a new method for preparing the 2-linear alkyl benzothiazole compound through the hydroheteroarylation reaction of the aliphatic alpha-olefin, which is realized by a divalent nickel catalyst.
Owner:SUZHOU UNIV
Method for preparing epoxide through induction of visible light
ActiveCN108084117AMild reaction conditionsGood substrate applicabilityOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsEpoxideOrganic synthesis
The invention belongs to the technical field of organic synthesis and provides a method for preparing an epoxide through induction of visible light. The method comprises the following step: under thecondition that the visible light and a photosensitizer exist, by taking oxygen or air as an oxygen source or an oxidizing agent and taking a synthesized amidine derivative as a catalyst, performing areaction at the temperature of -40-50 DEG C for 36h-192h, so that olefin is directly oxidized into the corresponding epoxide. The method is mild in reaction conditions, and the yield is as high as 94%or above, therefore, the method has good development value and application prospect.
Owner:DALIAN UNIV OF TECH
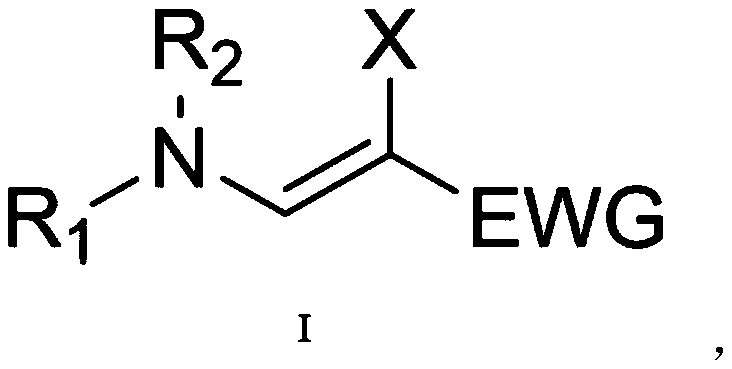
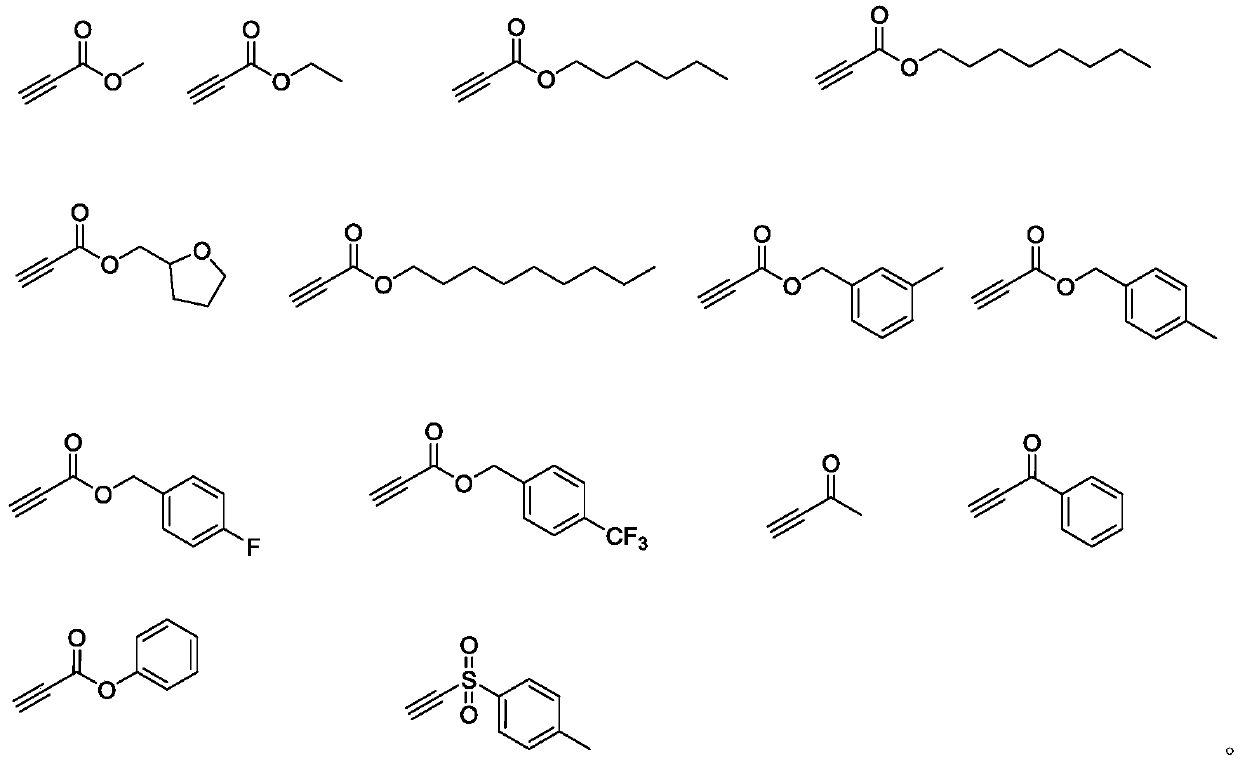
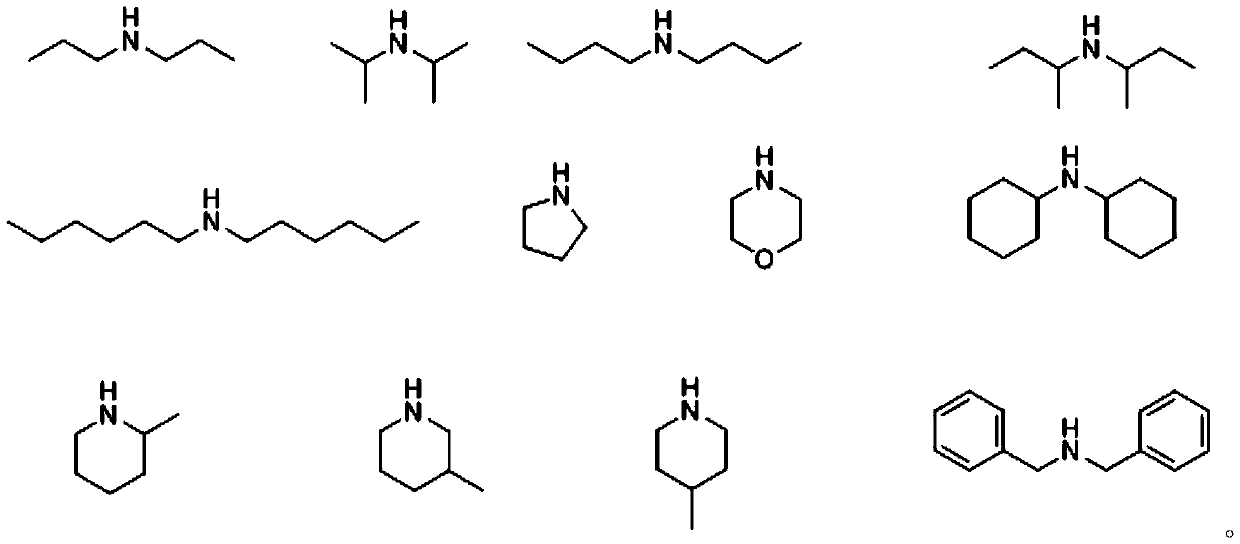
Beta-halogenated enamine acid ester compound and preparation method thereof
ActiveCN111423296AHigh reactivityHigh Synthetic AvailabilityOrganic compound preparationOrganic halogenationMorpholineEnamine
The invention discloses a beta-halogenated enamine acid ester compound and a preparation method thereof. The structure of the beta-halogenated enamine acid ester compound is shown as a formula I whichis described in the specification. In the formula I, R1 and R2 are selected from alkyl groups, morpholine, pyrrole, benzyl groups and hydrogen; X is Br or Cl; and EWG is an electron withdrawing group. The preparation method comprises the following steps: sequentially mixing dimethylformamide, an alkyne-terminated compound and secondary amine or derivatives thereof, carrying out stirring, mixing and sufficient reacting, adding triethylene diamine and N-halogenated imide, carrying out stirring and sufficient reacting at 0-50 DEG C, performing quenching with saturated edible salt water, and allowing a quenched product to pass through a column for purification so as to obtain the beta-halogenated enamine acid ester compound. According to the method, the enamine acid ester compound with high activity is prepared by utilizing metal catalysis-free multi-component reactions, operation is simple and convenient, assembly efficiency is high, automation can be easily realized, and the used raw materials are cheap and easy to obtain; and the method has the advantages of simple, mild and green reaction conditions and good substrate applicability, and can realize high yield in virtue of most amino compounds (especially the secondary amine).
Owner:JIANGSU UNIV OF SCI & TECH
Novel method for synthesizing benzimidazole[1,2-a]quinoline derivative
ActiveCN110143962AAddress reactivitySolve the difficulty of obtaining raw materialsOrganic chemistryQuinolineSolvent
The invention relates to a novel method for synthesizing a benzimidazole[1,2-a]quinoline derivative. An N-arylamidine compound and a benzisoxazole compound are used as raw materials, and an N1, N3-disubstituted imidazole ionic liquid is used as a solvent. Through a C-H activation / cyclization reaction catalyzed by a transition metal, a C-C bond is formed on the aromatic ring and then the benzimidazole[1,2-a]quinoline derivative is synthesized. In comparison with a traditional method, the method of the invention has the following advantages: (1) the step is simple, the raw materials are easily available, the substrate has a wide application range, and the reaction yield is high; and (2) addition of silver salt and an acid or alkali additive is avoided through the catalytic system, the cost is low, and the method is safe and convenient and has a wide application prospect.
Owner:SICHUAN UNIV
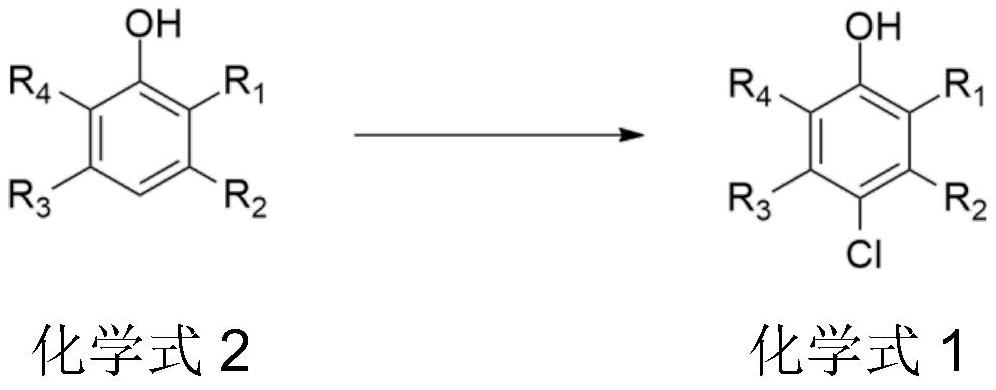
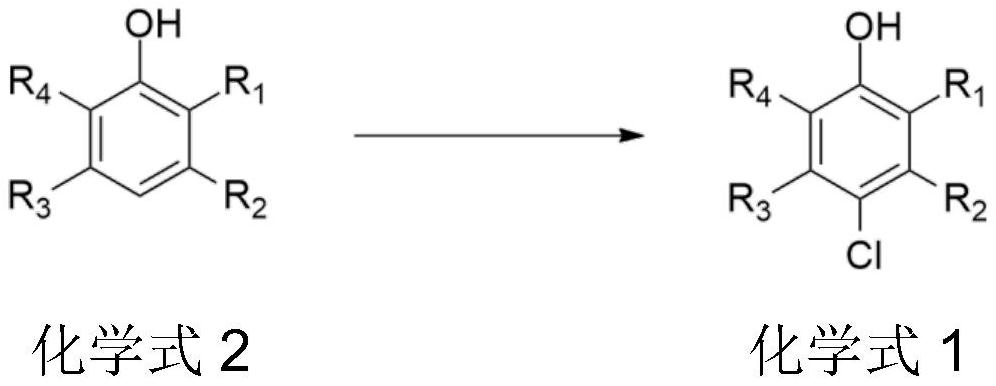
Synthesis method of chlorinated phenol compound
PendingCN114805033AAvoid it happening againHigh selectivityOrganic chemistryOrganic compound preparationSulfonyl chloridePtru catalyst
The invention provides a synthetic method of a chlorinated phenol compound, which takes Lewis acid series alkyl imidazole ionic liquid as a catalyst, and a phenol compound, hydrochloric acid and an oxidizing agent are subjected to an oxidation chlorination reaction to obtain the chlorinated phenol compound. Hydrochloric acid is used as a chlorinating agent, so that the use of chlorine, sulfonyl chloride and other environment-unfriendly chlorinating agents is avoided, and the method is green and environment-friendly; the Lewis acid series alkyl imidazole ionic liquid is adopted as the catalyst, no organic solvent is needed, the catalytic activity is high, the selectivity is high, product separation is facilitated, waste liquid is not generated, industrial production is facilitated, and the cost is reduced.
Owner:SHAANXI COAL & CHEM TECH INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

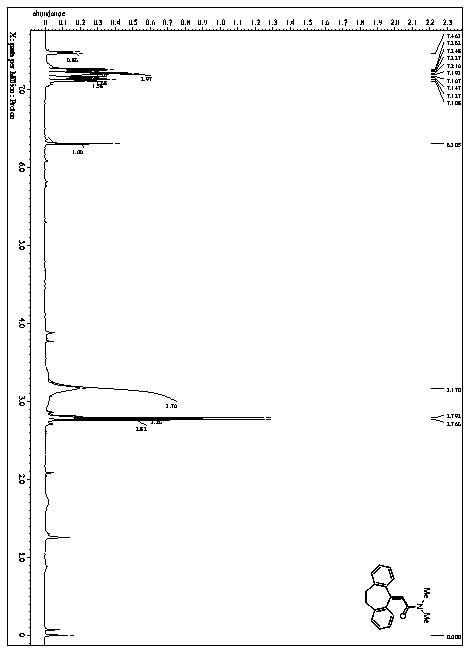


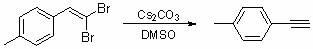


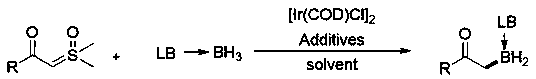

![Novel method for synthesizing benzimidazole[1,2-a]quinoline derivative Novel method for synthesizing benzimidazole[1,2-a]quinoline derivative](https://images-eureka.patsnap.com/patent_img/26d91266-bcb1-4756-863b-49f6f0d49397/190619093759.png)
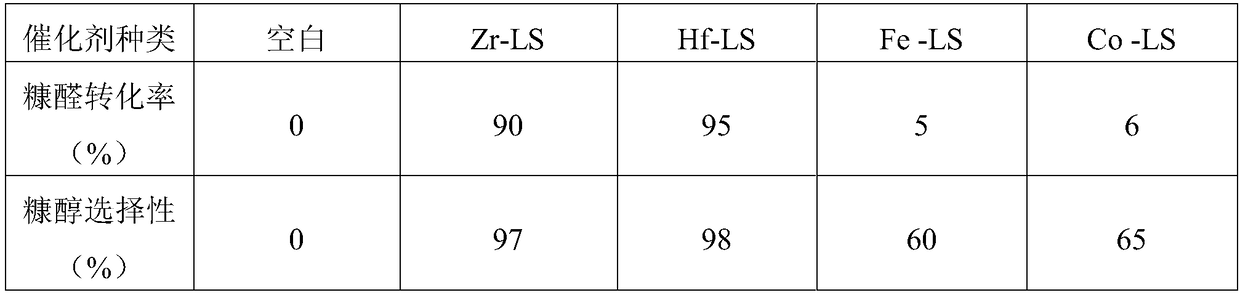
![Novel method for synthesizing benzimidazole[1,2-a]quinoline derivative Novel method for synthesizing benzimidazole[1,2-a]quinoline derivative](https://images-eureka.patsnap.com/patent_img/26d91266-bcb1-4756-863b-49f6f0d49397/190619093803.png)
![Novel method for synthesizing benzimidazole[1,2-a]quinoline derivative Novel method for synthesizing benzimidazole[1,2-a]quinoline derivative](https://images-eureka.patsnap.com/patent_img/26d91266-bcb1-4756-863b-49f6f0d49397/6253DEST_PATH_IMAGE001.png)