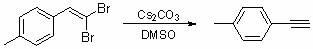Method for synthesizing end alkynyl by utilizing 1,1-two bromination vinyl compound
A technology of vinyl compounds and compounds, applied in the direction of preparing amino compounds from amines, organic chemical methods, chemical instruments and methods, etc., can solve the problems that the terminal alkyne compound method is not very convenient and economical, and achieve good substrate applicability, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: the synthesis of p-methylphenylacetylene
[0018]
[0019] Add 138 mg (0.5 mmol) of p-cresyl dibromide, 406 mg (1.25 mmol) of cesium carbonate, and 5 mL of dimethyl sulfoxide into the reactor, and stir the reaction at 95 ° C for 24 hours. After the reaction is complete, add deionized water to the system , extracted with ether. The organic layer was washed with saturated brine and dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain the crude product, which was then purified by column chromatography using petroleum ether as the eluent to obtain the desired product as a colorless liquid , 53mg, yield 92%.
[0020] Its NMR data are as follows:
[0021] 1 H-NMR (500MHz, CDCl 3 ): δ 2.34(s, 3H), 3.02(s, 1H), 7.11(d, J = 7.85 Hz, 2H), 7.37(d, J = 8.10 Hz, 2H).
Embodiment 2
[0022] Embodiment 2: the synthesis of p-chlorophenylacetylene
[0023]
[0024] Add 148 mg (0.5 mmol) of p-chlorostyryl dibromide, 207 mg (1.5 mmol) of potassium carbonate, and 5 mL of dimethyl sulfoxide into the reactor, and stir the reaction at 140°C for 12 hours. After the reaction is complete, add deionized water, extracted with ether. The organic layer was washed with saturated brine and dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain the crude product, which was then purified by column chromatography using petroleum ether as the eluent to obtain the desired product as a light yellow liquid , 60mg, yield 88%.
[0025] Its NMR data are as follows:
[0026] 1 H-NMR (500MHz, CDCl 3 ): δ 3.10(s, 1H), 7.28(d, J = 8.90 Hz, 2H), 7.40(d, J = 8.90 Hz, 2H).
Embodiment 3
[0027] Embodiment 3: the synthesis of phenylacetylene
[0028]
[0029] Add 131mg (0.5mmol) of styryl dibromide, 147mg (1.5mmol) of potassium acetate, 244mg (0.75mmol) of cesium carbonate, and 6mL of N,N-dimethylformamide into the reactor, and stir the reaction at 120°C for 10 After 1 hour, the reaction was complete, and deionized water was added to the system, followed by extraction with ether. The organic layer was washed with saturated brine and dried over anhydrous sodium sulfate, and the solvent was evaporated under reduced pressure to obtain the crude product, which was then purified by column chromatography using petroleum ether as the eluent to obtain the desired product as a colorless liquid , 44mg, yield 87%.
[0030] Its NMR data are as follows:
[0031] 1 H-NMR (500MHz, CDCl 3 ): δ3.06(s, 1H), 7.28-7.34(m, 3H), 7.48-7.50(m, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com