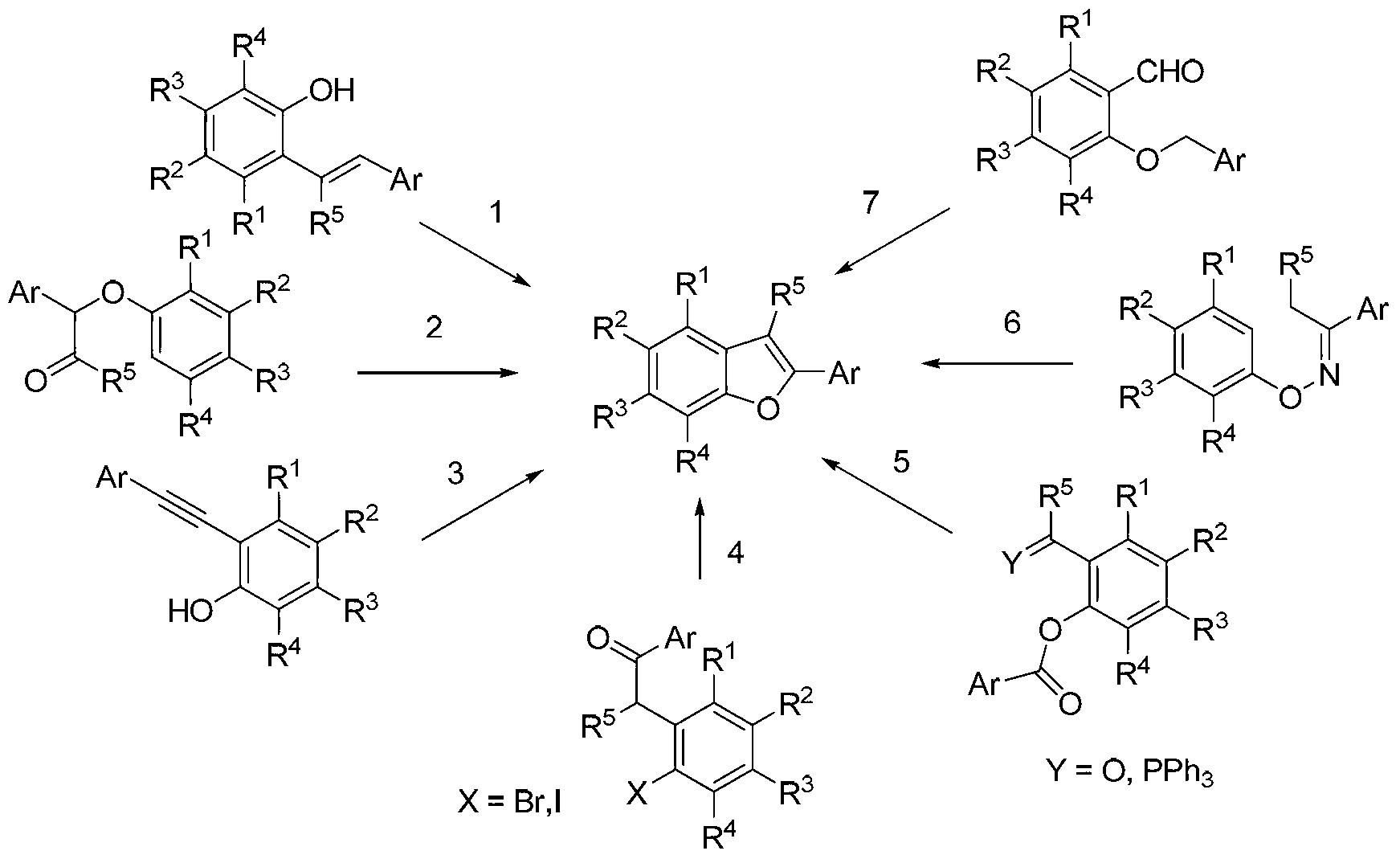
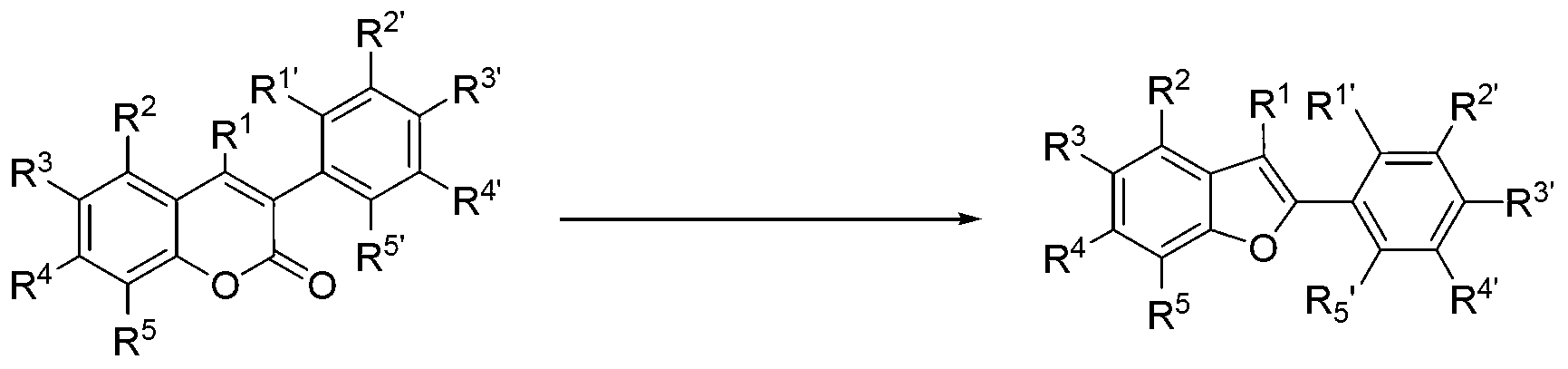
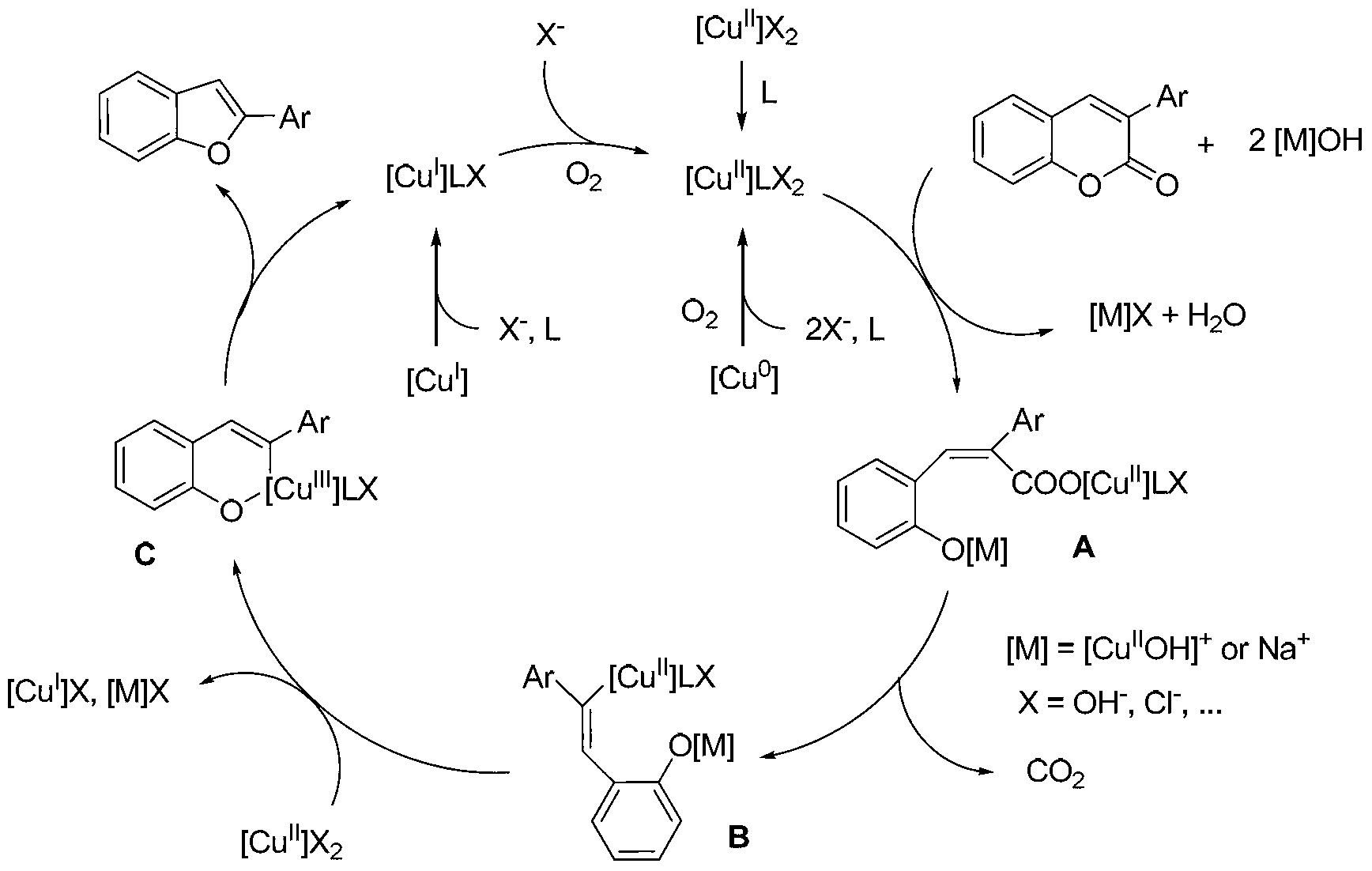
Synthesis method for 2-arylbenzofuran and derivative thereof
A derivative, arylbenzene technology, applied in the field of organic synthesis, can solve the problems of expensive catalyst, poor substrate adaptability, difficult raw material preparation, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] This example describes the synthesis test carried out in the reaction system A and in the preferred mode of staged temperature rise.
[0053] The reaction system includes: raw material (1 mmol), inorganic base, copper catalyst, ligand, and reaction medium. Raw material: inorganic base: copper catalyst: ligand: reaction medium=1:3:(0.1~10):0.15:5.
[0054] The raw materials specifically relate to 3-aryl coumarin and its derivatives a1-a10.
[0055] Reaction process: Add the reaction system to the reaction bottle, and add crushed molecular sieves (6 4A molecular sieves) at the same time, connect the drying tube; place the reaction bottle at a temperature of W°C and stir for X hours, then raise the temperature to a temperature of Y°C, and react for Z hours . Keep the system open to the atmosphere during the reaction process.
[0056] Extraction of the crude product: After the reaction, the reaction solution was adjusted to pH=2.0 with hydrochloric acid (6mol / L), extract...
Embodiment 2
[0062] This example describes the synthesis test carried out in the preferred mode of staged temperature rise with the reaction system B.
[0063] The reaction system includes: raw material (1 mmol), copper hydroxide or / and cuprous hydroxide, ligand, and reaction medium. Raw material: copper hydroxide or / and cuprous hydroxide: ligand: reaction medium=1: (2~10): (0.15~10): (5~100).
[0064] Synthesis and product extraction and purification operations are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com