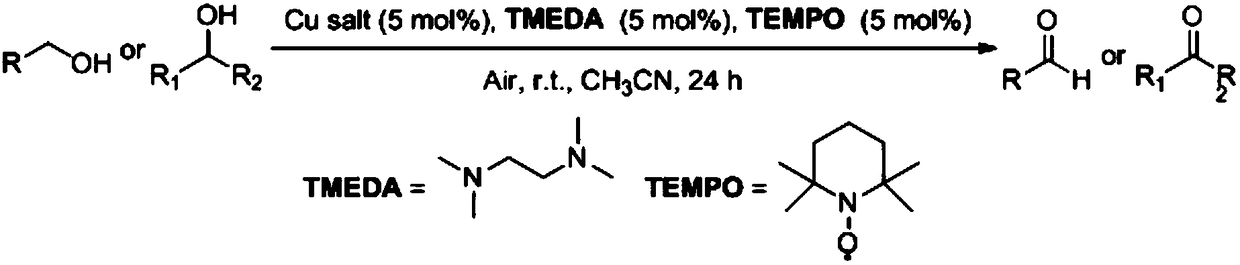
Method for preparing aldehyde or ketone by copper salt/bidentate ligand/TEMPO catalytic air oxidation alcohol
A bidentate ligand and alcohol oxidation technology, which is applied in the field of copper salt/bidentate ligand/TEMPO catalyzing air oxidation of alcohol to prepare aldehyde or ketone, can solve the problem of increasing the cost of catalytic reaction, inconsistency with green, energy saving and sustainable development, The system composition is complex and other problems, to achieve the effect of high yield, simple operation and simplified catalytic system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
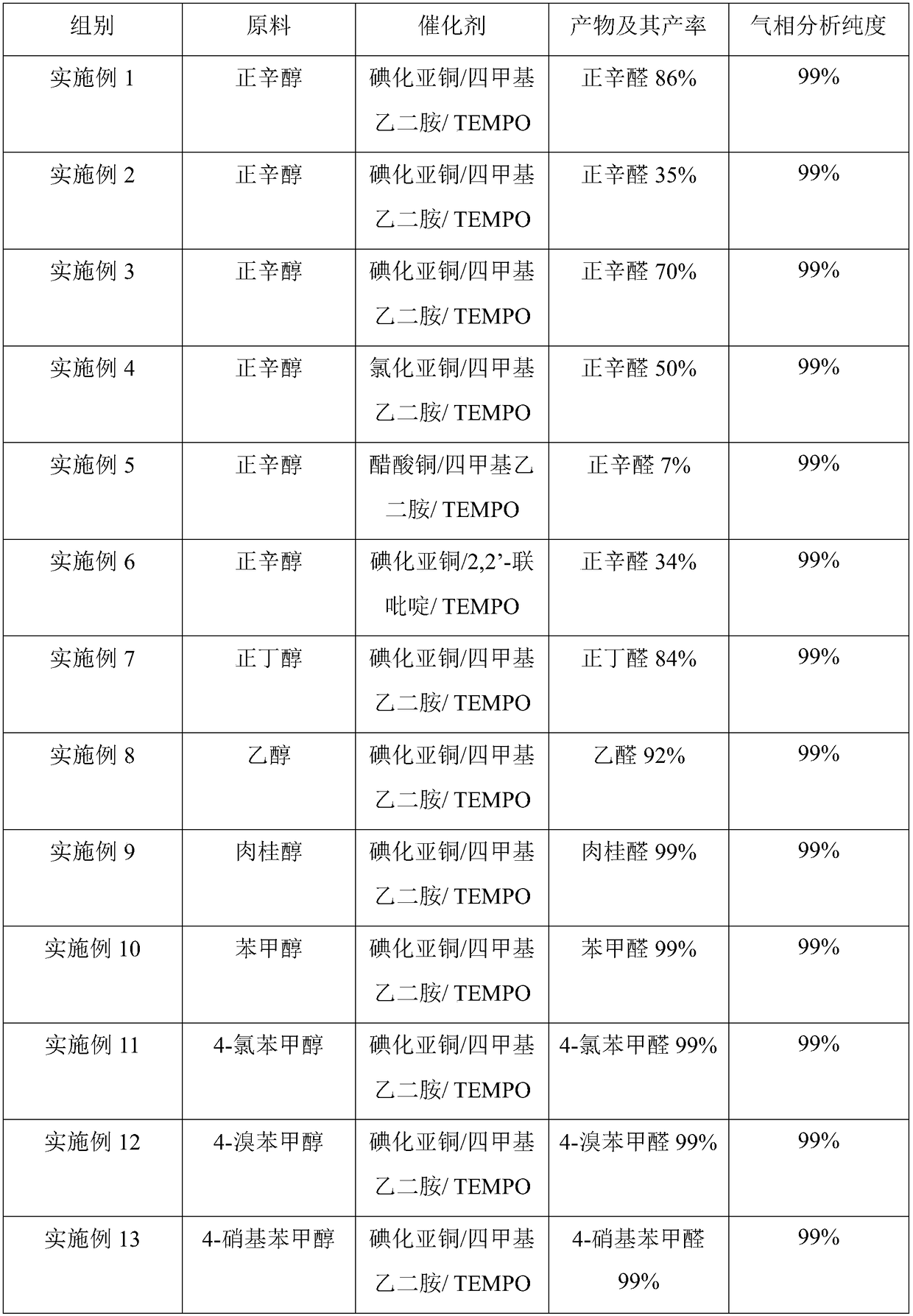
[0018] In a 25mL reaction flask, copper iodide (47.2mg, 0.25mmol) was added, followed by tetramethylethylenediamine (TMEDA) (29mg, 0.25mmol), TEMPO (39.1mg, 0.25mmol) and n-octanol ( 651mg, 5mmol), 5mL dichloromethane. Stir vigorously in air at 25 °C for 24 h. After 24 hours of reaction stop, pipette 0.5mL internal standard (biphenyl), then dichloromethane to a 10mL volumetric flask to volume, use an organic microporous filter membrane to filter, absorb 0.2μL of the filtered solution into the The product was analyzed by gas chromatography. According to the internal standard-standard curve method, the calculated yield of n-octanal was 86%, and the gas phase analysis purity was 99%.
[0019] Its chemical formula is as follows:
[0020]
Embodiment 2
[0022] To the 25mL reaction flask, add cuprous iodide (47.2mg, 0.25mmol), then add tetramethylethylenediamine (58mg, 0.5mmol), TEMPO (39.1mg, 0.25mmol) and n-octanol (651mg, 5mmol) ), 5 mL of dichloromethane. Stir vigorously in air at 25 °C for 24 h. After 24 hours of reaction stop, pipette 0.5mL internal standard (biphenyl), then dichloromethane to a 10mL volumetric flask to volume, use an organic microporous filter membrane to filter, absorb 0.2μL of the filtered solution into the The product was analyzed by gas chromatography. According to the internal standard-standard curve method, the calculated yield of n-octanal was 35%, and the gas phase analysis purity was 99%.
Embodiment 3
[0024] In a 25mL reaction flask, copper iodide (47.2mg, 0.25mmol) was added, followed by tetramethylethylenediamine (29mg, 0.25mmol), TEMPO (39.1mg, 0.25mmol) and n-octanol (651mg, 5mmol) ), 5mL acetonitrile. Stir vigorously in air at 25 °C for 24 h. After 24 hours of reaction stop, pipette 0.5mL internal standard (biphenyl), then dichloromethane to a 10mL volumetric flask to volume, use an organic microporous filter membrane to filter, absorb 0.2μL of the filtered solution into the The product was analyzed by gas chromatography. According to the internal standard-standard curve method, the calculated yield of n-octanal was 70%, and the gas phase analysis purity was 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com