Method for preparing aldehyde ketone by efficiently catalyzing molecular oxygen to oxidize alcohol by taking hydrotalcite-like material as catalyst
A technology for molecular oxygen oxidation of alcohols and catalysts, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds. It can solve the problems of high reaction efficiency, low conversion rate and selectivity, and achieve good substrates Applicability, high-efficiency catalytic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
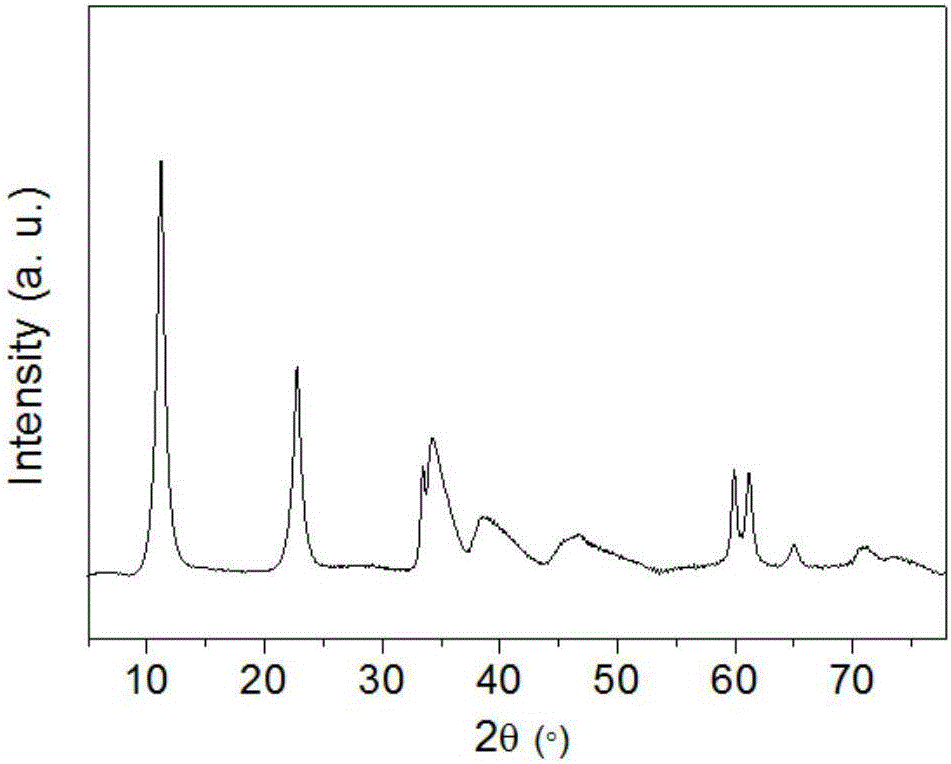
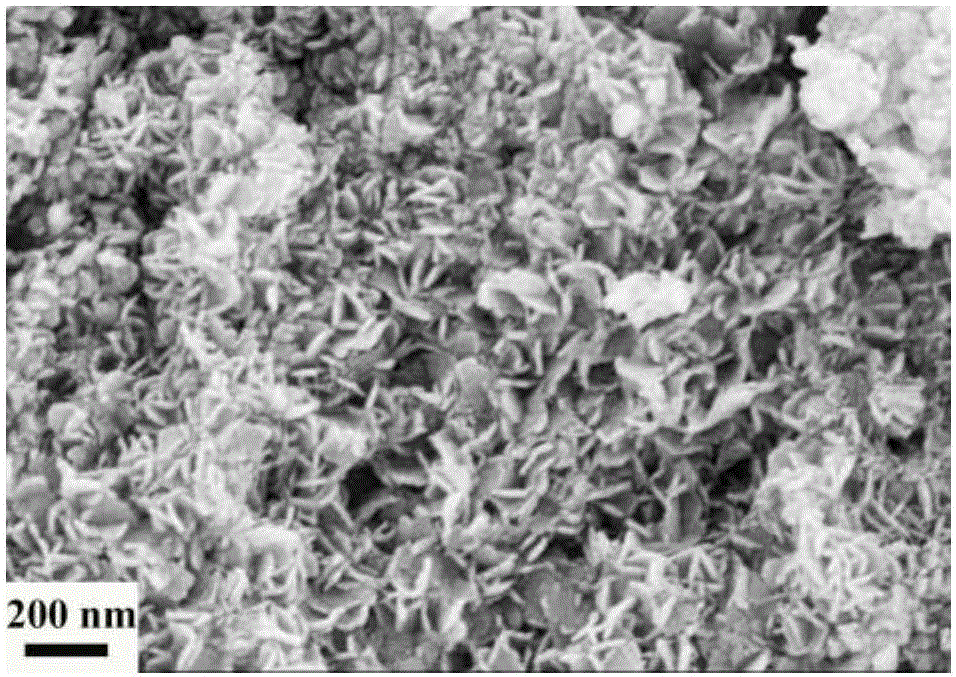
Image
Examples
Embodiment 1~3
[0016] Catalyst A for the preparation of aldehyde or ketone compounds by alcohol oxidation according to the present invention n– -Ni x M-LDHs can be prepared according to the following examples 1-3:
Embodiment 1
[0018] Prepare 200 mL of mixed salt solution of 0.16 mol / L nickel nitrate hexahydrate and 0.08 mol / L gallium nitrate, and 200 mL of mixed alkali solution of 0.2 mol / L sodium hydroxide and 0.2 mol / L ammonia water. in N 2 Under protection, slowly add the two mixed solutions prepared above dropwise to 100mL of water at 25°C. During the dropping process, stir vigorously, and control the pH value of the system to maintain within the range of 10.0±0.2. After the dropping is completed, continue to stir for 6 hours, then age at 70°C for 12 hours, filter with suction, and wash with deionized water until neutral , dried at 70°C for 24h, and ground into powder. get OH – -Ni 2 Ga-LDH samples.
Embodiment 2
[0020] Prepare 200 mL of mixed salt solution of 0.16 mol / L nickel nitrate hexahydrate and 0.08 mol / L gallium nitrate, and 200 mL of mixed alkali solution of 0.2 mol / L sodium hydroxide and 0.04 mol / L sodium carbonate. At 25°C, slowly add the two mixed solutions prepared above dropwise into 100mL of water at the same time. During the dropping process, stir vigorously, and control the pH value of the system to maintain within the range of 10.0±0.2. After the dropping is completed, continue to stir for 6 hours, then age at 70°C for 12 hours, filter with suction, and wash with deionized water until neutral , dried at 70°C for 24h, and ground into powder. get CO 3 2– -Ni 2 Ga-LDH samples.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com