Method for preparing epoxide through induction of visible light
A technology of epoxy and visible light, applied in chemical instruments and methods, chemical/physical processes, organic compounds/hydrides/coordination complex catalysts, etc., can solve rare problems and achieve mild and good reaction conditions Application and development value, good environmental friendliness effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
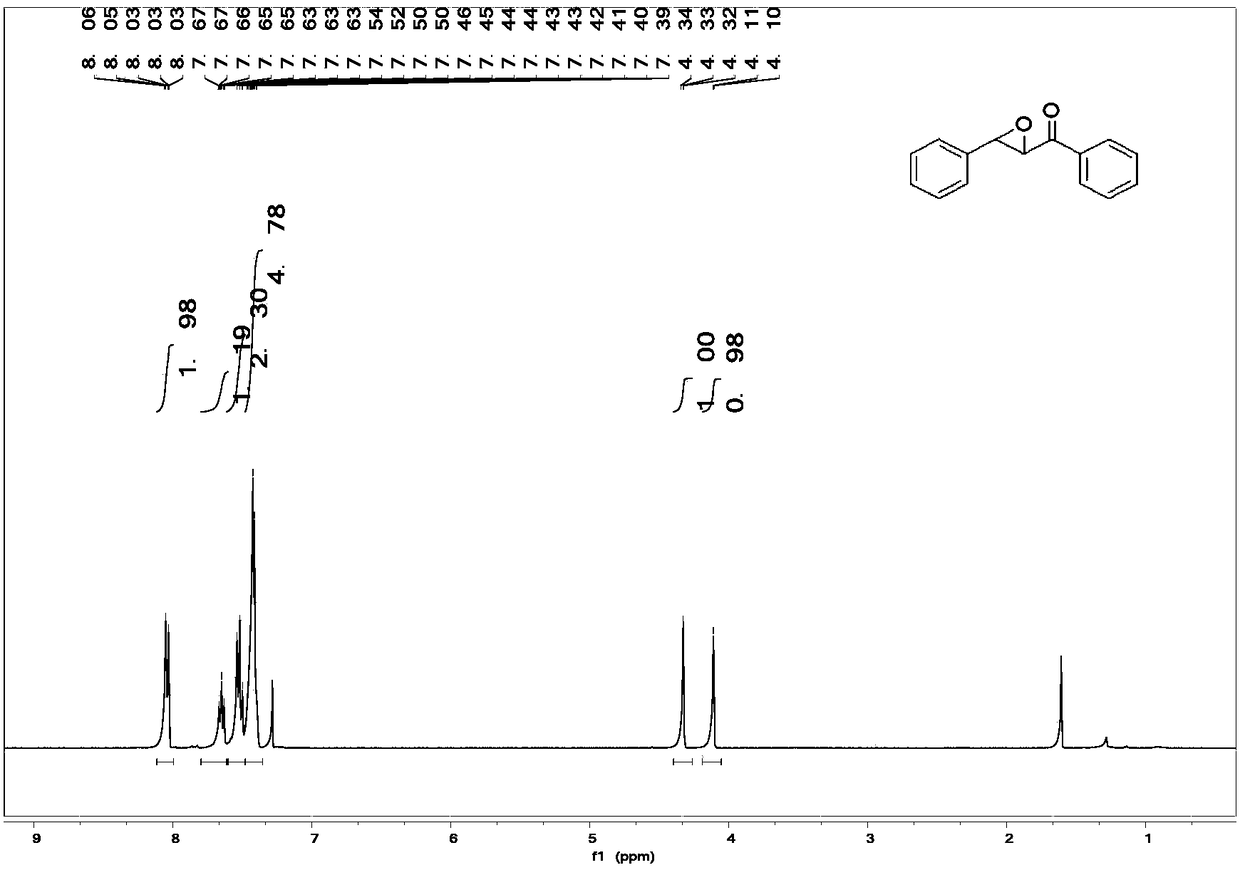
[0035] Example 1: Preparation of 1,3-diphenyl-2,3-epoxy-1-propanone
[0036] Weigh 1,3-diphenyl-2-propen-1-one (84.69mg, 0.4mmol) and tetraphenylporphyrin 1.62mg (0.0025mmol) into a 20ml single-port reaction tube, add toluene 2ml, IIIa- 1 (243 mg, 1.2 mmol). At 10°C, under an oxygen atmosphere, irradiate with a 10W LED light strip, and stir the reaction. After 96h, the reaction was completed, column separation, rotary evaporation, and vacuum drying gave the epoxidized product (35.34mg, 38% yield) 1 H NMR (400MHz, Chloroform-d) δ8.03 (t, J = 1.3Hz, 1H), 8.01 (d, J = 1.5Hz, 1H), 7.66–7.59 (m, 1H), 7.50 (m, J = 8.4,7.1Hz,2H),7.40(m,J=5.8,5.1,3.5Hz,5H),4.31(d,J=1.8Hz,1H),4.08(d,J=1.9Hz,1H).
Embodiment 2
[0037] Example 2: Preparation of 1,3-diphenyl-2,3-epoxy-1-propanone
[0038] Weigh 1,3-diphenyl-2-propen-1-one (82.98mg, 0.4mmol) and tetraphenylporphyrin (1.52mg, 0.0025mmol) into a 20ml single-port reaction tube, add toluene 2ml, IIIa -2 (556.8mg, 4mmol), at 10°C, under an oxygen atmosphere, irradiated with a 10W LED light strip, and stirred for reaction. After 144 hours, the reaction was completed, and the epoxidized product (58.74 mg, 66% yield) was obtained by column separation, rotary evaporation, and vacuum drying.
Embodiment 3
[0039] Example 3: Preparation of 1,3-diphenyl-2,3-epoxy-1-propanone
[0040] Weigh 1,3-diphenyl-2-propen-1-one (83.28mg, 0.4mmol) and tetraphenylporphyrin (1.50mg, 0.0025mmol) into a 20ml single-port reaction tube, add toluene 2ml, IIIa -2 (556.8mg, 4mmol), at 10°C, under an oxygen atmosphere, irradiated with a 10W LED light strip, and stirred for reaction. After 144 hours, the reaction was completed, and the epoxidized product (55.38 mg, 62% yield) was obtained by column separation, rotary evaporation, and vacuum drying.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com