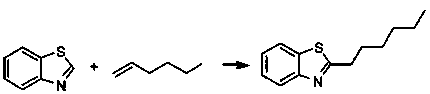Application of N-heterocyclic carbene-based mixed nickel (II) complex in reaction of synthesizing 2-linear alkylbenzothiazole compound
A technology for straight-chain alkylbenzenes and compounds, applied in the field of organic synthesis and preparation, can solve problems such as the inability to provide 2-linear alkylbenzothiazole compounds, achieve 100% atom economy, and catalysts are cheap and easy to obtain , the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
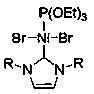
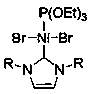
[0034] Example 1 See CN108002966A, Catalyst nitrogen heterocyclic carbene mixed type nickel (II) complex Ni[P(OEt) 3 ][(RNCHCHNR)C]Br 2 The chemical structural formula of is as follows, for the following examples:
[0035]
[0036] R has the following structural formula:
[0037] .
Embodiment 2
[0038] Example 2 The divalent nickel (II) complex is used as a catalyst to catalyze the hydroheteroarylation reaction of 1-hexene and benzothiazole
[0039]
[0040] Under argon protection, catalyst (66 mg, 0.05 mmol, 10 mol%), magnesium chips (6.0 mg, 0.25 mmol), benzothiazole (54 μl, 0.5 mmol), 1 -Hexene (93 microliters, 0.75 mmol), tetrahydrofuran (1.5 milliliters) as solvent, at 60 o The reaction was carried out at C for 3 hours, and the reaction was terminated with water. The reaction product was extracted with ethyl acetate and purified by column chromatography (with a mixed solvent of ethyl acetate / petroleum ether volume ratio of 1:10 as the developing solvent), and the yield was 95%.
[0041] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 ): δ 7.98 (d, J = 8.3 Hz, 1H), 7.85 – 7.80 (m, 1H),7.45 (ddd, J = 8.3, 7.2, 1.3 Hz, 1H), 7.38 –...
Embodiment 3
[0044] Example 3 The divalent nickel (II) complex is used as a catalyst to catalyze the hydroheteroarylation reaction of 1-heptene and benzothiazole
[0045] Under argon protection, catalyst (66 mg, 0.05 mmol, 10 mol%), magnesium chips (6.0 mg, 0.25 mmol), benzothiazole (54 μl, 0.5 mmol), 1 -heptene (106 microliters, 0.75 mmol), tetrahydrofuran (1.5 milliliters) as solvent, at 60 o The reaction was carried out at C for 3 hours, and the reaction was terminated with water. The reaction product was extracted with ethyl acetate, and purified by column chromatography (using a mixed solvent with a volume ratio of ethyl acetate / petroleum ether of 1:10 as the developing solvent), and the yield was 94%.
[0046] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 ): δ 7.97 (d,J = 8.3 Hz, 1H), 7.87 – 7.80 (m, 1H),7.46 (ddd, J = 8.3, 7.2, 1.3 Hz, 1H), 7.40 – 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com