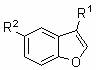
Method for synthesizing alpha-benzyl benzofuran compound
A technology of benzofuran and compounds, which is applied in the field of organic synthesis and preparation, can solve the problems of unseen substrate expansion, etc., and achieve the effect of easy synthesis and good substrate applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
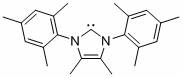
[0032] Embodiment one: Ni[P(OEt) 3 ]{[R`NC(CH 3 )C(CH 3 )NR]C}Br 2 (R` = 2,4,6-trimethylphenyl) synthesis
[0033] Under argon protection, nitrogen heterocyclic carbene [R`NC(CH 3 )C(CH 3 )NR`]C (0.3325 g, 1.0 mmol) was added to the tetrahydrofuran solution of bis(triethylphosphite)nickel(II) bromide (0.5508 g, 1.0 mmol), reacted at room temperature for 2 hours, and removed in vacuo Solvent, wash the residue with n-hexane, extract the residue with toluene, transfer the clear liquid and remove the solvent toluene to obtain a red solid that is a mixed nickel (II) complex with a yield of 85%, which is used as a catalyst for the following implementation For example, catalyzing the hydroheteroarylation reaction of aryl vinyl compounds and benzofuran compounds to prepare the product α-benzylbenzofuran compounds; and the catalyst does not change color in the air for two days, which can prove that the catalyst of the present invention Good stability in air.
[0034] The product...
Embodiment 2
[0042] Example 2 The divalent nickel (II) complex is used as a catalyst to catalyze the hydroheteroarylation reaction of styrene and benzofuran
[0043] Under the protection of argon, the catalyst (35.9 mg, 0.05 mmol, 10 mol%), sodium tert-butoxide (48 mg, 0.5 mmol), and benzofuran (55 μl, 0.5 mmol) were sequentially added to the reaction flask , styrene (86 μl, 0.75 mmol), toluene (1.5 ml) as solvent, at 110 o The reaction was carried out at C for 48 hours, and the reaction was terminated with water. The reaction product was extracted with ethyl acetate, separated and purified by column chromatography (using petroleum ether as a developing agent), and the yield was 92%. The product structural formula was as follows:
[0044]
[0045] Dissolve the product in CDCl 3Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 ): δ 7.61 (dd, J = 7.4, 1.9 Hz, 1H), 7.51 (dd, J =6.9, 2.3 Hz, 1H...
Embodiment 3
[0048] Example 3 The divalent nickel (II) complex is used as a catalyst to catalyze the hydroheteroarylation reaction of o-methoxystyrene and benzofuran
[0049] Under the protection of argon, the catalyst (35.9 mg, 0.05 mmol, 10 mol%), sodium tert-butoxide (48 mg, 0.5 mmol), and benzofuran (55 μl, 0.5 mmol) were sequentially added to the reaction flask , o-methoxystyrene (100 μl, 0.75 mmol), toluene (1.5 ml) as solvent, at 110 o The reaction was carried out at C for 48 hours, and the reaction was terminated with water. The reaction product was extracted with ethyl acetate, separated and purified by column chromatography (petroleum ether was used as the developing solvent), and the yield was 90%.
[0050] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 ): δ 7.63 – 7.57 (m, 1H), 7.49 (d, J = 7.8 Hz, 1H),7.35 – 7.27 (m, 3H), 7.21 (dd, J = 7.8, 1.7 Hz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com