Nitrogen-heterocyclic carbene mixed nickel(ii) complexes and their applications
A technology of nitrogen heterocycles and complexes, which is applied in the field of organic synthesis and preparation, can solve the problems of expensive catalysts, avoid the use of external ligands, and achieve the effects of single and clear catalyst structure and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
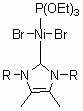
[0033] Embodiment one: Ni[P(OEt) 3 ]{[RNC(CH 3 )C(CH 3 )NR]C}Br 2 (R = 2,4,6-trimethylphenyl) synthesis
[0034] Under argon protection, nitrogen heterocyclic carbene [RNC(CH 3 )C(CH 3 )NR]C (0.3325 g, 1.0 mmol) was added to a tetrahydrofuran solution of di(triethylphosphite)nickel(II) bromide (0.5508 g, 1.0 mmol), reacted at room temperature for 3 hours, and removed the solvent in vacuo , wash the residue with n-hexane, extract the residue with toluene, transfer the supernatant and remove the solvent toluene to obtain a red solid as a divalent nickel (II) complex with a yield of 87%.
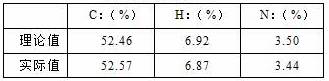
[0035] The product was subjected to elemental analysis, and the results are shown in Table 1:
[0036]
[0037] The product was characterized by NMR, and the results are as follows:
[0038]Dissolve the product in C 6 D. 6 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 ): δ 7.06 (s, 4...
Embodiment 2
[0039] Embodiment two: Ni[P(OEt) 3 ]{[RNC(CH 3 )C(CH 3 )NR]C}Br 2 (R = 2,6-Diisopropylphenyl) Synthesis
[0040] Under argon protection, nitrogen heterocyclic carbene [RNC(CH 3 )C(CH 3 )NR]C (0.4167 g, 1.0 mmol) was added to a tetrahydrofuran solution of bis(triethylphosphite) nickel(II) bromide (0.5508 g, 1.0 mmol), reacted at room temperature for 3 hours, and removed the solvent in vacuo , wash the residue with n-hexane, extract the residue with toluene, transfer the clear liquid and remove the solvent toluene to obtain a red solid as a divalent nickel (II) complex with a yield of 85%.
[0041] Carry out elemental analysis to product, the result is shown in the following table:
[0042]
[0043] The product was characterized by NMR, and the results are as follows:
[0044] Dissolve the product in C 6 D. 6 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 ): δ 7.53 (t,...
Embodiment 3
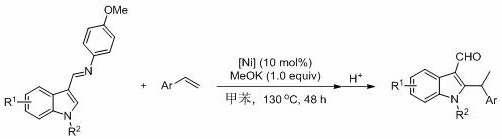
[0045] Embodiment three: Ni[P(OEt) 3 ]{[RNC(CH 3 )C(CH 3 )NR]C}Br 2 (R = 2,4,6-trimethylphenyl)-catalyzed hydroheteroarylation of N-methylindole-3-formaldehyde imine with styrene
[0046] Under the protection of argon, the catalyst (35.9 mg, 0.05 mmol, 10 mol%), potassium methylate (35.1 mg, 0.5 mmol), N-methylindole-3-formaldehyde imine (132.2 mg, 0.5 mmol), styrene (86 microliters, 0.75 mmol), toluene (1.5 ml) as solvent, at 130 o C for 48 hours, stop the reaction with water, add dilute hydrochloric acid (2 mol / L, 1 ml) for acidification, the reaction product is extracted with ethyl acetate, separated and purified by column chromatography (the volume ratio of ethyl acetate / petroleum ether is 1:5 mixed solvent as developer), the yield was 95%.
[0047] Dissolve the product in CDCl 3 Medium (about 0.4 mL), seal the tube, measure and characterize on a Unity Inova-400 NMR instrument at room temperature: 1 H NMR (400 MHz, CDCl 3 ): δ 10.31 (s, 1H), 8.43 (dd, J = 6.1, 2....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com