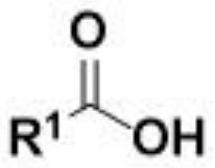
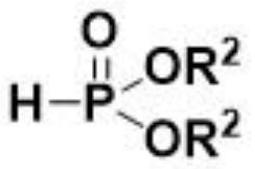
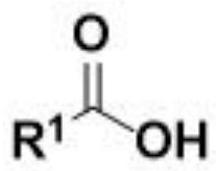
Method for electrochemical synthesis of acyl phosphate
An acyl phosphate, electrochemical technology, applied in the field of electrochemical synthesis of acyl phosphate, can solve the problems of poor operability, unfriendly environment, limited reaction substrates, etc., and achieve strong operability, air stability, The effect of simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Phosphorylation of p-toluic acid 3a by diethyl phosphite.
[0031] Use p-toluic acid 1a (27.2 mg, 0.2 mmol), diethyl phosphite 2a (77.2 μL, 0.6 mmol), tetrabutylammonium bromide (66.5 mg, 1 equiv.), and platinum electrodes as positive and negative electrodes, respectively , added in 8.0mL of acetonitrile, at room temperature, the system reacted under the condition of a continuous current of 10mA for 6h in an air atmosphere and then stopped the reaction, separated by column chromatography (using a silica gel column; eluent: sherwood oil / ethyl acetate=7 / 1) to obtain the phosphorylated product 3a of p-toluic acid. The product was a colorless liquid with a yield of 90%.
[0032] 1 HNMR (400MHz, CDCl 3 )δ7.87(d, J=7.6Hz, 2H), 7.21(d, J=7.5Hz, 2H), 4.29(p, J=7.2Hz, 4H), 2.36(s, 3H), 1.34(t, J=7.1Hz, 6H). 13 C NMR (101MHz, CDCl 3 )δ162.01(d, J=8.4Hz), 147.62, 132.62, 130.45, 126.35(d, J=8.1Hz), 66.21(d, J=5.9Hz), 22.72, 17.02(d, J=6.6Hz) .
[0033] Its synt...
Embodiment 2
[0035] Example 2: Phosphorylation of benzoic acid by diethyl phosphite 3b.
[0036] Use p-toluic acid 1b (24.4 mg, 0.2 mmol), diethyl phosphite 2a (77.2 μL, 0.6 mmol), tetrabutylammonium bromide (66.5 mg, 1 equiv.), and platinum electrodes as positive and negative electrodes, respectively , added in 8.0mL of acetonitrile, at room temperature, the system reacted under the condition of a continuous current of 10mA for 6h in an air atmosphere and then stopped the reaction, separated by column chromatography (using a silica gel column; eluent: sherwood oil / ethyl acetate=7 / 1), obtain the phosphorylation product 3b of benzoic acid. The product was a colorless liquid with a yield of 85%.
[0037] 1 HNMR (400MHz, CDCl 3 )δ8.09(d, J=7.1Hz, 2H), 7.67(t, J=7.7Hz, 1H), 7.51(t, J=7.9Hz, 2H), 4.51-4.29(m, 4H), 1.45( td, J=7.2, 0.9Hz, 6H). 13 C NMR (101MHz, CDCl 3 )δ160.87(d, J=8.2Hz), 134.45, 130.43, 128.6, 7128.08(d, J=8.3Hz), 65.15(d, J=5.9Hz), 16.02(d, J=6.9Hz).
[0038] Its synt...
Embodiment 3
[0040] Example 3: Phosphorylation of o-toluic acid by diethyl phosphite 3c.
[0041] Use o-toluic acid 1c (27.2 mg, 0.2 mmol), diethyl phosphite 2a (77.2 μL, 0.6 mmol), tetrabutylammonium bromide (66.5 mg, 1 equiv.), and platinum electrodes as positive and negative electrodes, respectively , added in 8.0mL of acetonitrile, at room temperature, the system reacted under the condition of a continuous current of 10mA for 6h in an air atmosphere and then stopped the reaction, separated by column chromatography (using a silica gel column; eluent: sherwood oil / ethyl acetate=7 / 1) to obtain the phosphorylated product 3c of o-toluic acid. The product was a colorless liquid with a yield of 70%.
[0042] 1 HNMR (400MHz, CDCl3 )δ8.01(d, J=7.3Hz, 1H), 7.51(t, J=6.9Hz, 1H), 7.41(d, J=7.2Hz, 2H), 4.56-4.29(m, 4H), 2.67( s, 3H), 1.45 (t, J=6.7Hz, 6H). 13 C NMR (101MHz, CDCl 3 )δ160.91(d, J=8.1Hz), 142.55, 133.69, 132.12, 131.72, 126.62(d, J=7.9Hz), 125.89, 65.12(d, J=5.9Hz), 21.91, 16.05...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com