Synthesis method and application of cage compounds
A clathrate compound and synthesis method technology, applied in the field of catalysis, can solve the problems of accelerating the oxidation rate of petroleum distillate, large amount of solvent medium, low yield of petroleum sulfoxide, etc., and achieve simple post-treatment process, simple preparation method, and product purity high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
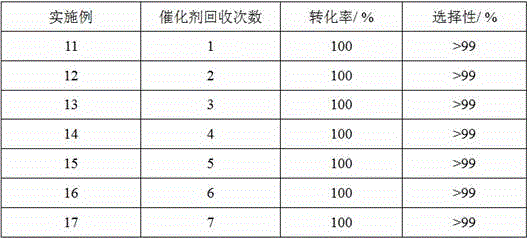
Examples
Embodiment 1
[0029] Example 1. Synthesis of Ni(II)-Salen(CHO) ligand with catalytic function
[0030] 1) Add 3.84 g of 5-bromo-3-tert-butyl salicylaldehyde and 0.45 g of ethylenediamine to a 100 mL three-necked flask in sequence, then add 70 mL of ethanol, and react at 70 °C for 24 h under the protection of an inert gas. After cooling to 15 °C, the reaction solution was concentrated to 20 mL by rotary evaporator at 40 °C, and then allowed to stand at -30 °C for 3 h, suction filtered with a Buchner funnel, and the filter cake was washed with 10 mL ice ethanol After 5 passes, the filter cake was collected to obtain 3.46 g of the Salen(Br) ligand, with a yield of 86%. 1 H NMR (CDCl 3 , 400MHz) δ(ppm): 1.41[s, 18 H, C(CH 3 ) 3 ], 3.94(s, 4H), 7.20(s, 2H), 7.37(s, 2H), 8.29(d, J=1.6 Hz, 2H), 13.82(s, 2H, OH);
[0031] 2) Add 3.0 g Salen(Br) ligand, 1.53 g Ni(OAc) to a 100 mL three-necked flask in sequence 2 4H 2 O and 60mL chloroform / methanol (1:1, v:v) solution, and then reacted at 85 °C...
Embodiment 2
[0033] Embodiment 2. Synthesis of Ni(II)-Salen(CHO) ligand with catalytic function
[0034] 1) Add 3.84 g of 5-bromo-3-tert-butyl salicylaldehyde and 0.45 g of ethylenediamine to a 250 mL three-necked flask in sequence, then add 150 mL of ethanol, and react at 100 °C for 12 h under the protection of an inert gas. After cooling to 30 °C, the reaction solution was concentrated to 10 mL by rotary evaporator at 70 °C, then allowed to stand at -5 °C for 5 h, suction filtered with a Buchner funnel, and the filter cake was washed with 20 mL of ice ethanol for 3 After several times, the filter cake was collected to obtain 3.37 g of Salen(Br) ligand, the yield of which was 84%. 1 H NMR (CDCl 3 , 400MHz) δ(ppm): 1.43[s, 18 H, C(CH 3 ) 3 ], 3.92(s, 4H), 7.18(s, 2H), 7.35(s, 2H), 8.29(d, J=1.6 Hz, 2H), 13.80(s, 2H, OH);
[0035] 2) Add 3.0 g of Salen(Br) ligand and 1.50 g of NiCl to a 100 mL three-necked flask in sequence 2 ·6H 2 O and 60 mL of dichloromethane / methanol (1:1, v:v) so...
Embodiment 3
[0037] Example 3. Synthesis of cage compounds
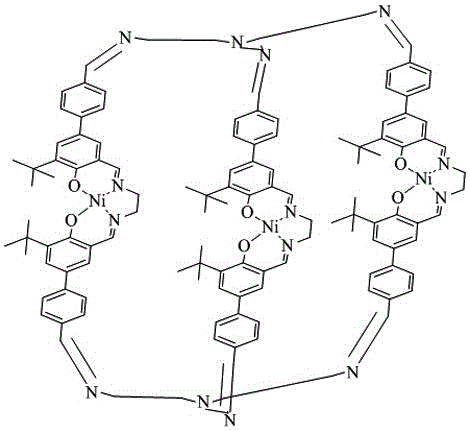
[0038] Dissolve 200 mg Ni(II)-Salen(CHO) in tetrahydrofuran, add 13.8 μL tris(2-aminoethyl)amine under nitrogen atmosphere, heat and reflux for 8 h, and remove the solvent by rotary evaporation after the reaction is complete, the obtained solid After washing with methanol and drying in vacuo, 178 mg of the clathrate compound was obtained with a yield of 81%, and its single crystal was obtained by recrystallization. High resolution mass spectrometry (HR-MS, ESI): [C 126 h 138 N 10 o 6 Ni] + m / z calculated: 2120.9075; experimental: 2120.8988.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com