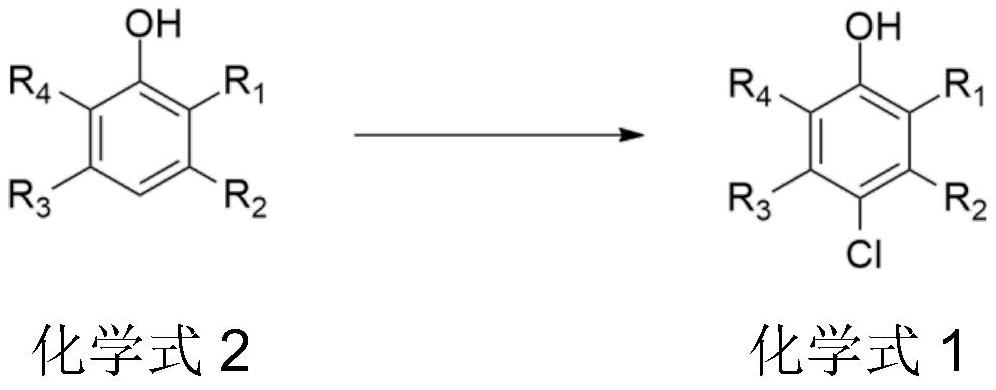
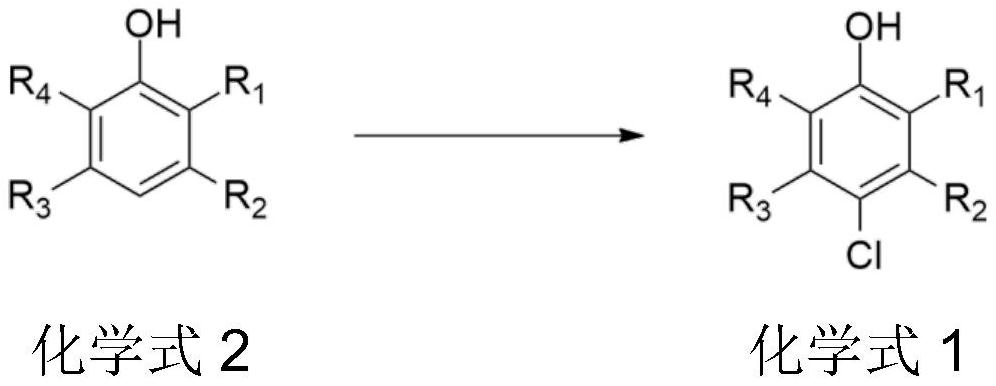
Synthesis method of chlorinated phenol compound
A technology of phenolic compounds and chlorinated phenols, which is applied in the field of green catalytic synthesis of chlorinated phenolic compounds, can solve the problems of large catalyst ratio, reduced reaction efficiency, unfavorable industrialization, etc., and achieves high selectivity, efficient catalytic reaction, and separation convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1 Synthesis of alkyl imidazole ionic liquid
[0028] 1) The preparation process of 1-allyl-3-methylimidazole ionic liquid is as follows:
[0029] 200mL of allyl chloride and 132mL of methylimidazole were added to a 500mL flask respectively, magnetically stirred at 60°C, refluxed for 10h, cooled down and then rotary-evaporated, and the excess allyl chloride was spun out to obtain 1-allyl group -3-methylimidazolium chloride, which is light yellow transparent liquid with a yield of about 92%;
[0030] 2) The preparation process of 1-butyl-3-methylimidazolium ionic liquid is as follows:
[0031] 142mL of n-chlorobutane and 100mL of methylimidazole (molar ratio 1.1:1) were added to a 500mL flask, refluxed at 100°C for 48h, magnetically stirred, and then rotary-evaporated to obtain a light yellow transparent liquid, which was cooled to White solid in 95% yield.
[0032]The preparation process of Lewis acid alkyl imidazolium ionic liquid is as follows:
[0033] ...
Embodiment 2
[0046] 15g (0.122mol) 3,5-dimethylphenol, 30g CuCl 2 / 1-allyl-3-methylimidazolium chloride ionic liquid complex was put into a 100mL four-necked flask, and 15.74mL of hydrochloric acid (mass fraction 37%) was added, and the reaction was carried out by introducing oxygen at 70 ° C for 9 hours, The conversion rate is greater than 96%, and the selectivity is greater than 93%. After the reaction was completed, suction filtration was performed, and the obtained crude product was recrystallized with tetrachloroethylene to obtain 16.5 g of pure 4-chloro-3,5-dimethylphenol with a purity of 99.3%, and the yield was 86%.
[0047]
Embodiment 3
[0049] The CuCl isolated after the reaction of Example 2 is used in Example 3 2 / 1-allyl-3-methylimidazolium ionic liquid, Examples 4-8 sequentially use the CuCl separated after the reaction in the previous example 2 / 1-allyl-3-methylimidazole ionic liquid, the effect of repeated recycling on the reaction is shown in Table 1.
[0050] Table 1. CuCl 2 / 1-allyl-3-methylimidazolium ionic liquid cycle times and conversion rate, yield comparison
[0051]
[0052]
[0053] It can be seen from Table 1 that the CuCl2 / 1-allyl-3-methylimidazolium ionic liquid catalyst still has good catalytic activity after being recycled for 6 times, and the conversion rate and yield are relatively stable. In industrial production, It is beneficial to reduce the cost and avoid the generation of waste liquid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com