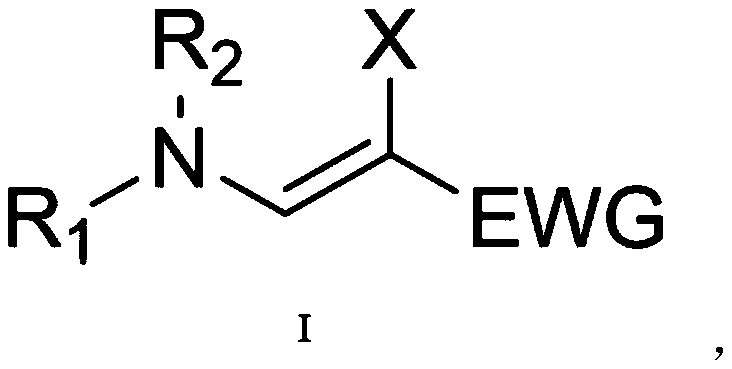
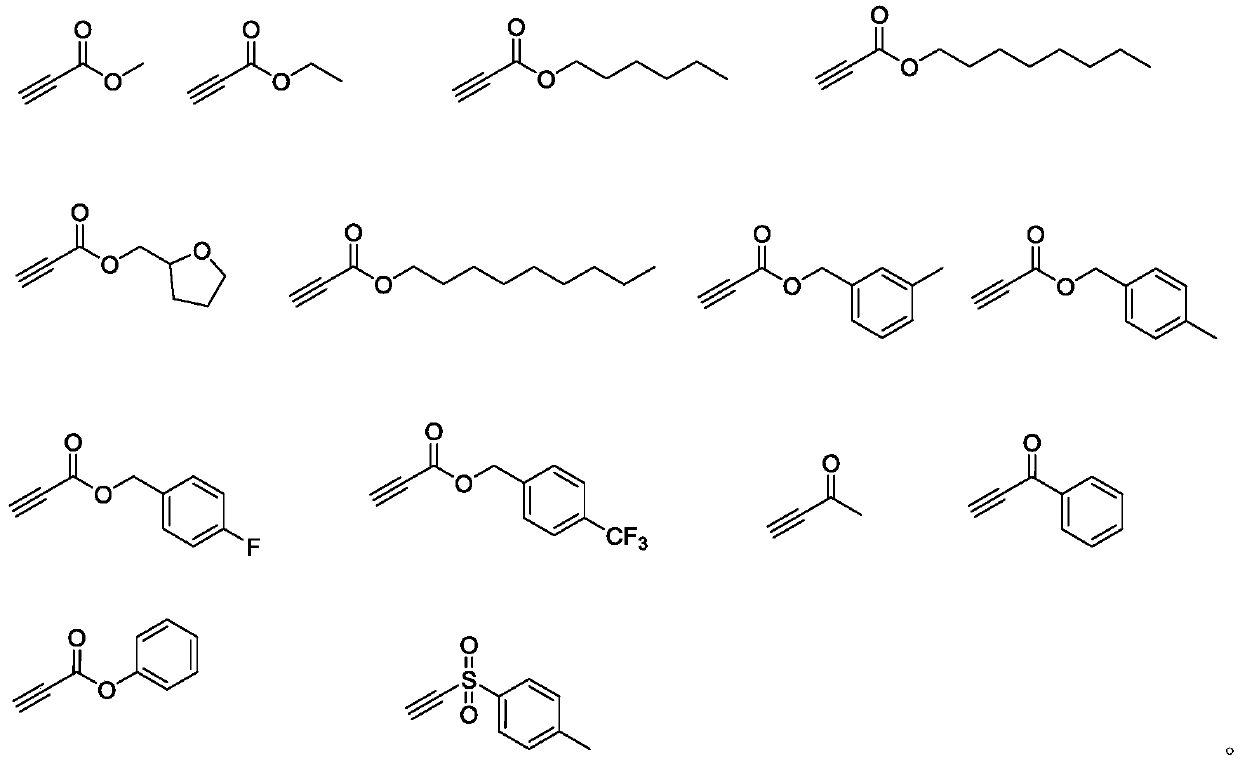
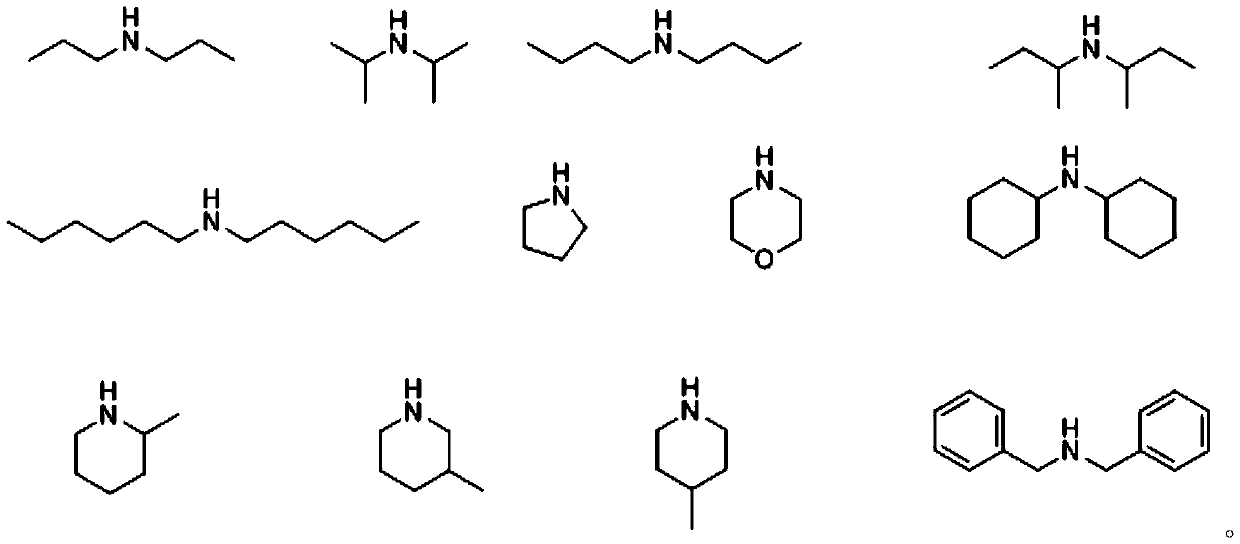
Beta-halogenated enamine acid ester compound and preparation method thereof
A technology of haloenaminoate esters and compounds, which is applied in the field of β-haloenaminoate ester compounds and its preparation, can solve the problems of insufficient active groups and functional application limitations, and achieve simple reaction conditions and high Synthetic availability, effects of good substrate suitability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: Synthesis of β-bromoeninoate compound ethyl(Z)-2-bromo-3-(dipropylamino)acrylate
[0026]
[0027] A polytetrafluoroethylene magnet was placed in a dry 15 mL pressure-resistant tube, and then DMF (1 mL), ethyl propiolate (51 μL, 0.5 mmol) and di-n-propylamine (103 μL, 0.75 mmol) were sequentially added. After the reaction mixture was stirred at room temperature for 10 min, DABCO (112.12 mg, 1 mmol) and NBS (177.98 mg, 1 mmol) were added thereto. The reaction mixture was stirred at room temperature for 2 h, then quenched with 15 mL of saturated brine; then extracted with ethyl acetate (10 mL×3 times), the organic phases were combined, and dried over anhydrous sodium sulfate; Go out ethyl acetate, obtain crude product; This crude product is purified with neutral alumina chromatographic column, with V 石油醚 :V 乙酸乙酯 =10:1 as the developer, the pure product was obtained as light yellow oil with a yield of 81%.
[0028] Identification data of the product of thi...
Embodiment 2
[0030] Example 2: Synthesis of β-bromoeninoate compound ethyl(Z)-2-bromo-3-morpholinoacrylate
[0031]
[0032] A polytetrafluoroethylene magnet was placed in a dry 15 mL pressure-resistant tube, and then DMF (1 mL), ethyl propiolate (51 μL, 0.5 mmol) and morpholine (66 μL, 0.75 mmol) were sequentially added. After the reaction mixture was stirred at room temperature for 10 min, DABCO (112.12 mg, 1 mmol) and NBS (177.98 mg, 1 mmol) were added to the solution. The reaction mixture was stirred at 50° C. for 4 h, then quenched with 15 mL of saturated brine; then extracted with ethyl acetate (10 mL×3 times), and the organic phases were combined and dried over anhydrous sodium sulfate. Ethyl acetate was spun off using a rotary evaporator to obtain the crude product. This crude product is purified with neutral alumina chromatographic column, with V 石油醚 :V 乙酸乙酯 =4:1 was used as the developer, and the pure product was obtained as a white solid with a yield of 68%.
[0033] Iden...
Embodiment 3
[0035] Example 3: Synthesis of β-bromoenidinate compound ethyl(Z)-2-bromo-3-(pyrrolidin-1-yl)acrylate
[0036]
[0037]A polytetrafluoroethylene magnet was placed in a dry 15 mL pressure-resistant tube, and then DMF (1 mL), ethyl propiolate (51 μL, 0.5 mmol) and tetrahydropyrrole (66 μL, 0.75 mmol) were sequentially added. After the reaction mixture was stirred and reacted in an ice-water bath for 10 min, DABCO (112.12 mg, 1 mmol) and NBS (177.98 mg, 1 mmol) were further added thereto. The reaction mixture was stirred at 0° C. for 0.5 h, then quenched with 15 mL of saturated brine; then extracted with ethyl acetate (10 mL×3 times), and the organic phases were combined and dried over anhydrous sodium sulfate. Ethyl acetate was spun off using a rotary evaporator to obtain the crude product. This crude product is purified with neutral alumina chromatographic column, with V 石油醚 :V 乙酸乙酯 =10:1 as developing solvent, the pure product was obtained as pale yellow oil with a yield...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com