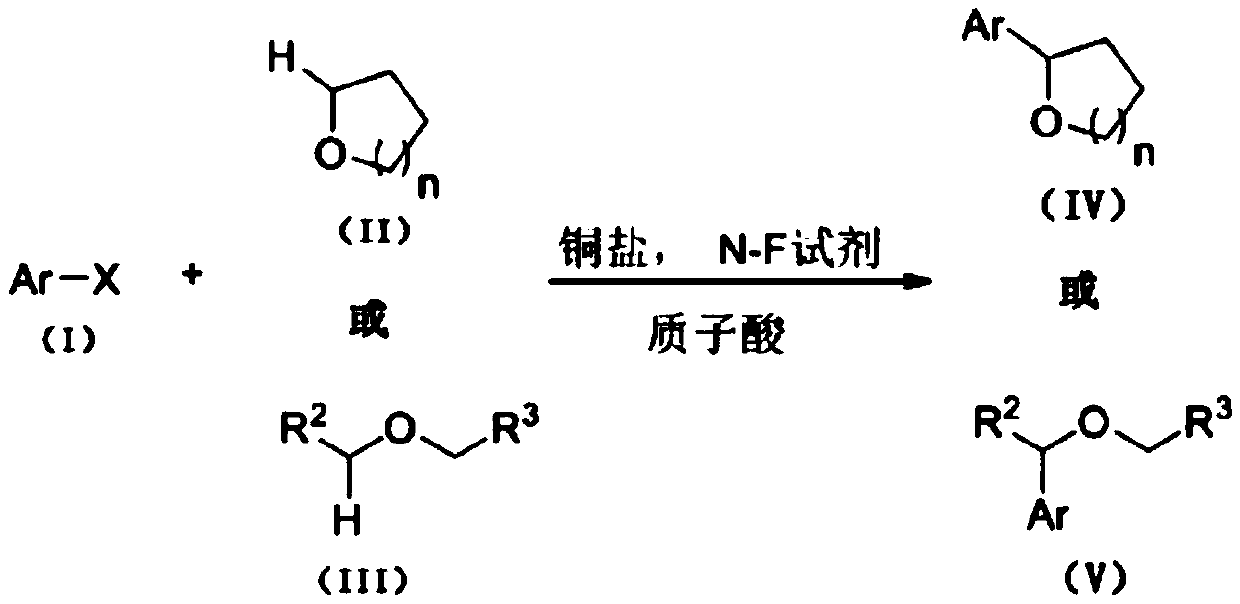
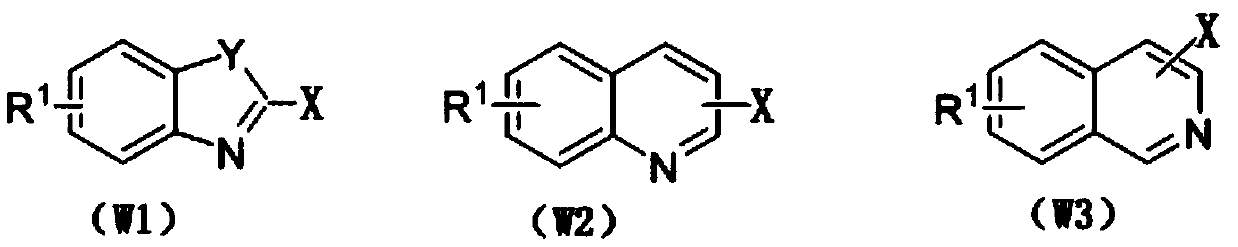
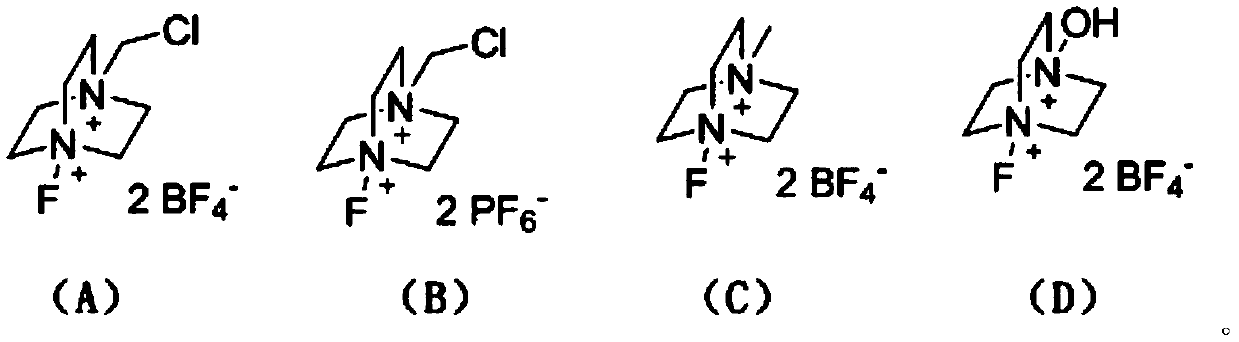
Method for preparing N-heterocyclic aromatic hydrocarbon derivatives by dehalogenation and alkylation
A technology for heterocyclic aromatic hydrocarbons and alkylation, which is applied in the field of preparing N-heterocyclic aromatic hydrocarbon derivatives, can solve the problems of difficulty in achieving high regioselectivity, few synthesis methods, etc., and achieves wide application range of substrates and cheap reagents. , mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 4,7-dichloroquinoline (0.5mmol, 99mg), tetrahydrofuran (10mmol, 720mg), CuBr (0.025mmol, 3.6mg), selective fluorine reagent (1mmol, 354.3mg) and trifluoromethanesulfonic acid (0.5mmol , 75 mg) was added to a 5 mL single-port reaction flask, acetonitrile (5.0 mL) was added as a solvent, and the oil bath was heated to 50° C. for 5 h. After the reaction, the reaction solution was washed with water and extracted with ethyl acetate, and then separated into an organic layer and an aqueous layer. The organic layer was dried over anhydrous sodium sulfate and concentrated by distillation under reduced pressure to obtain a yellow oil. The yellow oil was separated by column chromatography, using a mixture of petroleum ether and ethyl acetate with a volume ratio of 10:1 as the eluent, the eluate containing the target compound was collected, the solvent was evaporated and dried to obtain 87.5 mg of light yellow oil 7 -Chloro-4-(tetrahydrofuran-2-yl)quinoline, the yield is 75%, and i...
Embodiment 2
[0034] The protonic acid in the system was replaced with trifluoroacetic acid (0.5mmol, 57mg), and other operating conditions were the same as in Example 1, and finally 70.3mg of light yellow oily substance 7-chloro-4-(tetrahydrofuran-2-yl)quinoline was obtained. Yield 60%.
Embodiment 3
[0036] The THF dosage was changed to 2.5 mmol, and the other operating conditions were the same as in Example 1 to finally obtain 60.6 mg of light yellow oil 7-chloro-4-(tetrahydrofuran-2-yl)quinoline with a yield of 52%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com