Preparation method of substituted urea compound
A technology for substituted urea and compounds, which is applied in the field of preparation of substituted urea compounds, can solve the problems of low atom economy, high toxicity of intermediate products, and non-environmental protection, and achieve the advantages of wide source of raw materials, simple and easy operation methods, and reduced environment effect of influence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Add 15.59 parts of tetrachloromethane (99.0% content) into a reactor containing 85.00 parts of trifluorotoluene, add 38.01 parts of 1-tert-butoxycarbonylpiperazine (98.0% content), and then add 0.31 parts of trioxide Manganese (99.5%) and 32.48 parts of cesium carbonate (content is 99.9%), under xenon lamp as light source irradiation, after stirring reaction 10 hours, collect the chlorine that reaction process generates, the brown oily solution distillation that obtains is reclaimed trifluorotoluene solvent, A caramel-colored viscous 1,1'-carbonylbis(4-tert-butoxycarbonylpiperazine) liquid product was obtained.
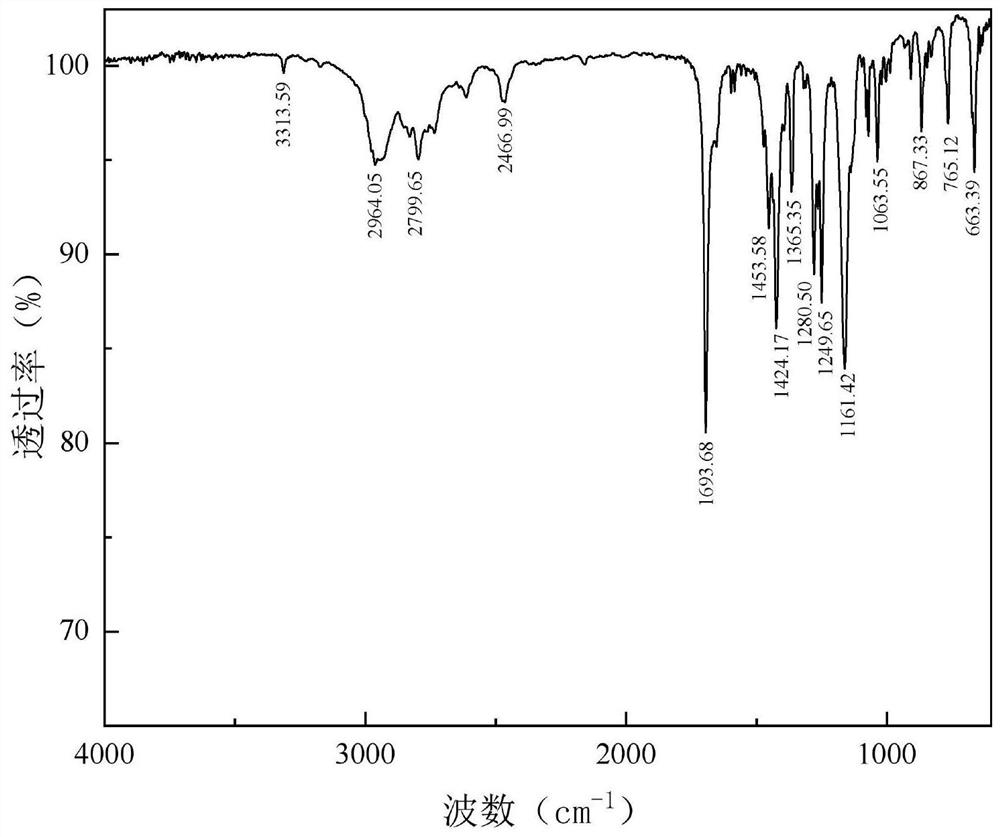
[0044] After separation and purification of the obtained product, structural characterization was carried out by infrared spectroscopy and mass spectrometry. The results are as follows: figure 1 and figure 2 Shown:
[0045] The infrared spectrum of the 1,1'-carbonyl bis(4-tert-butoxycarbonylpiperazine) obtained in Example 1 is as follows: figure 1 As shown, i...
Embodiment 2
[0047] 15.54 parts of tetrachloromethane (content is 99.0%) are added in the reactor that 85.00 parts of benzotrifluoride are housed, add 20.23 parts of 1-methylpiperazine (content is 99.0%), then add 0.32 parts of trimanganese tetraoxide ( 99.5%) and 33.35 parts of cesium carbonate (content is 99.9%), under the irradiation of xenon lamp as the light source, after stirring for 10 hours, the chlorine gas generated in the reaction process was collected, and the obtained oily solution was distilled to reclaim the trifluorotoluene solvent to obtain light yellow Bis(4-methylpiperazin-1-yl)methanone solid product.
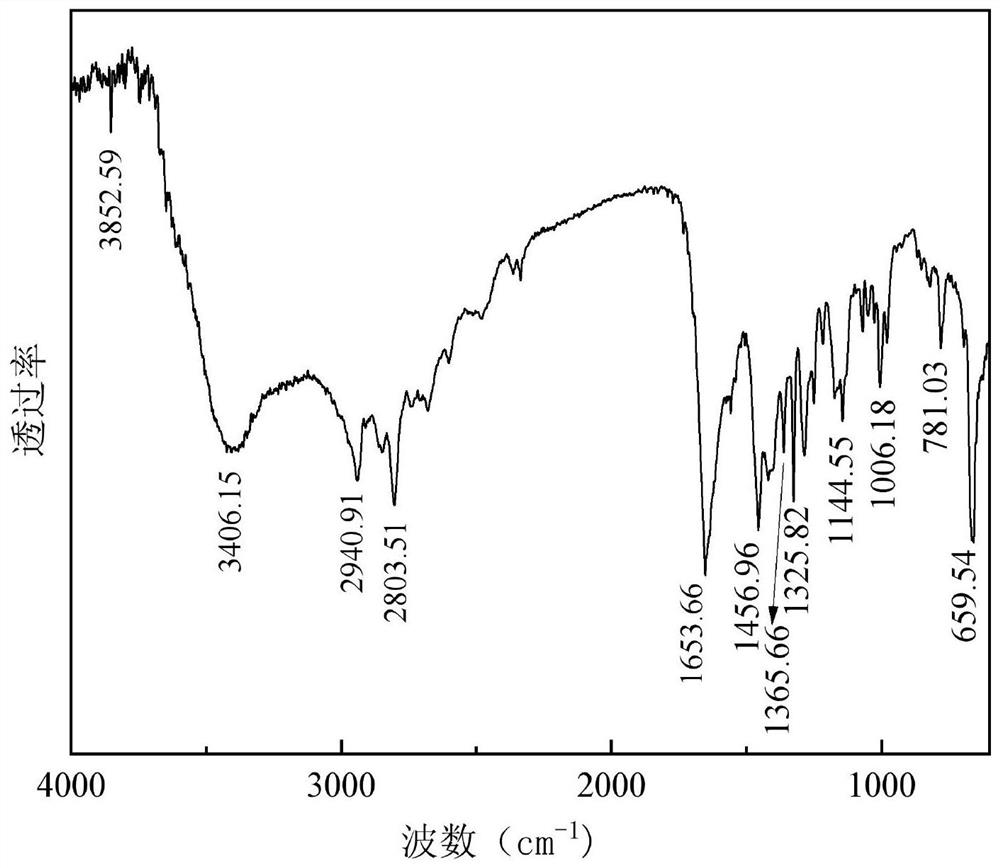
[0048] After separating and purifying the obtained product, the structure was characterized by infrared spectroscopy, the results are as follows: image 3 Shown:
[0049] The infrared spectrum of the two (4-methylpiperazin-1-yl) ketone that embodiment 2 obtains is as follows image 3 As shown, its main characteristic peaks are (cm -1 ): 3406.15 attributed to N-H stret...
Embodiment 3
[0051] 34.25 parts of tetrabromomethane (content is 97.0%) are added in the reactor that 85.00 parts of benzotrifluoride are housed, add 33.11 parts of 1-phenylpiperazine (content is 99.0%), then add 0.30 parts of trimanganese tetraoxide (99.5 %) and 32.07 parts of cesium carbonate (content is 99.9%), under xenon lamp as light source irradiation, stirring reaction after 10 hours, leave standstill layering, upper strata is yellow liquid, and lower stratum is the bromine simple substance of red black oily state, and supernatant solution is distilled The trifluorotoluene solvent was recovered to obtain a reddish-brown 1,1'-carbonylbis(4-phenylpiperazine) solid product.
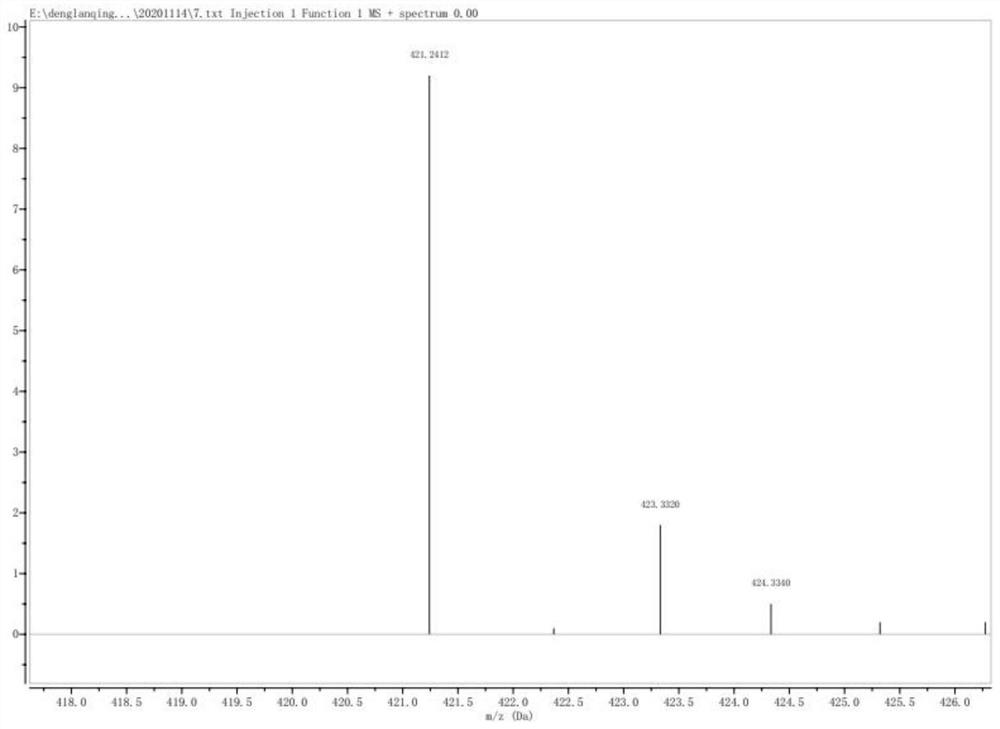
[0052] After separating and purifying the obtained product, the structure was characterized by mass spectrometry, the results are as follows: Figure 4 Shown:
[0053] The mass spectrum of the 1,1'-carbonyl bis(4-phenylpiperazine) obtained in Example 3 is as follows Figure 4 As shown, the peak with a mass-to-c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com