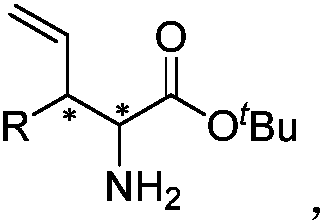
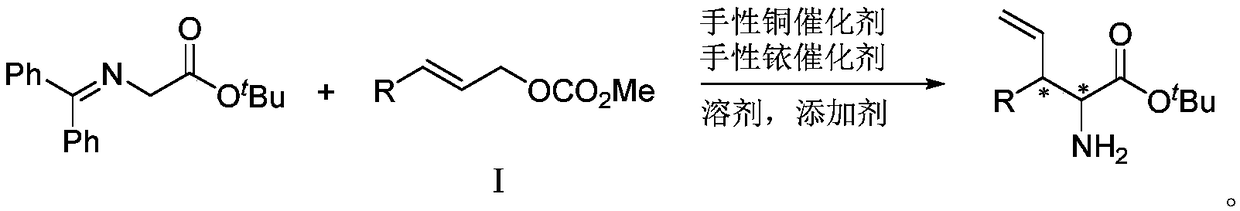
Chiral 3-substituted 3-vinyl-2-amino propionate and preparation method thereof
An aminopropionate and vinyl technology, which is applied in the field of 3-substituted 3-vinyl-2-aminopropionate and its preparation, and achieves the effects of mild reaction conditions, simple operation and strong substrate applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 5.4 mg (R, R, R)-L1 ligand and 3.36 mg (1,5-cyclooctadiene) iridium chloride dimer to a 5 ml reaction bottle, and add 0.5 ml Tetrahydrofuran and 0.5ml of n-propylamine were stirred at 50°C for 30 minutes, and the solvent was concentrated and spin-dried to obtain an iridium catalyst.
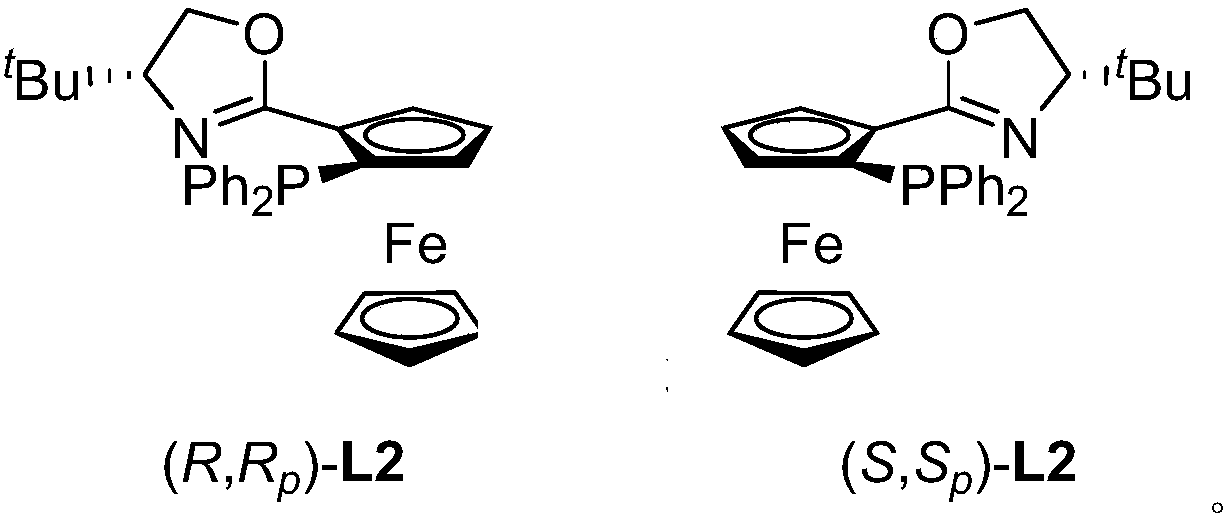
[0034] Add 4.5mg copper trifluoromethanesulfonate, 6.2mg (S, S p )-L2 ligand, under the condition of nitrogen protection, 1ml of tetrahydrofuran was added thereto, and stirred at 25°C for 30 minutes to obtain a copper catalyst.
[0035] Under nitrogen protection conditions, 48.0mg cinnamyl methyl carbonate, 65.7mg tert-butyl benzylidene aminoacetate, copper catalyst, iridium catalyst and 2ml tetrahydrofuran were added to a 10ml reaction flask, stirred at 30°C for 12 hours. Concentrate the reaction solution to dry the solvent, pass through a silica gel column to obtain the purified product (2S,3R)-2-amino-3-phenyl-4-pentenoic acid tert-butyl ester, the structural formula is as follows,...
Embodiment 2
[0039] Add 5.4 mg (R, R, R)-L1 ligand and 3.36 mg (1,5-cyclooctadiene) iridium chloride dimer to a 5 ml reaction bottle, and add 0.5 ml Tetrahydrofuran and 0.5ml of n-propylamine were stirred at 50°C for 30 minutes, and the solvent was concentrated and spin-dried to obtain an iridium catalyst.
[0040] Add 4.5mg copper trifluoromethanesulfonate, 6.2mg (S, S p )-L2 ligand, under the condition of nitrogen protection, 1ml of tetrahydrofuran was added thereto, and stirred at 25°C for 30 minutes to obtain a copper catalyst.
[0041] Under nitrogen protection, 58.5mg cinnamyl tert-butyl carbonate, 88.6mg dibenzylidene glycinate tert-butyl ester, copper catalyst, iridium catalyst and 2ml tetrahydrofuran were added to a 10ml reaction flask, and stirred at 30°C for 12 hours. Concentrate the reaction solution to dry the solvent, pass through a silica gel column to obtain the purified product (2S,3R)-2-amino-3-phenyl-4-pentenoic acid tert-butyl ester, the structural formula is as follow...
Embodiment 3
[0044] Add 5.4 mg (R, R, R)-L1 ligand and 3.36 mg (1,5-cyclooctadiene) iridium chloride dimer to a 5 ml reaction bottle, and add 0.5 ml Tetrahydrofuran and 0.5ml of n-propylamine were stirred at 50°C for 30 minutes, and the solvent was concentrated and spin-dried to obtain an iridium catalyst.
[0045] Add 4.5mg copper trifluoromethanesulfonate, 6.2mg (S, S p )-L2 ligand, under the condition of nitrogen protection, 1ml of tetrahydrofuran was added thereto, and stirred at 25°C for 30 minutes to obtain a copper catalyst.
[0046] Under nitrogen protection conditions, 48.0mg cinnamyl methyl carbonate, 88.6mg dibenzylidene glycinate tert-butyl ester, copper catalyst, iridium catalyst and 2ml tetrahydrofuran were added into a 10ml reaction flask, and stirred at 30°C for 12 hours. Concentrate the reaction solution to dry the solvent, pass through a silica gel column to obtain the purified product (2S,3R)-2-amino-3-phenyl-4-pentenoic acid tert-butyl ester, the structural formula is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com