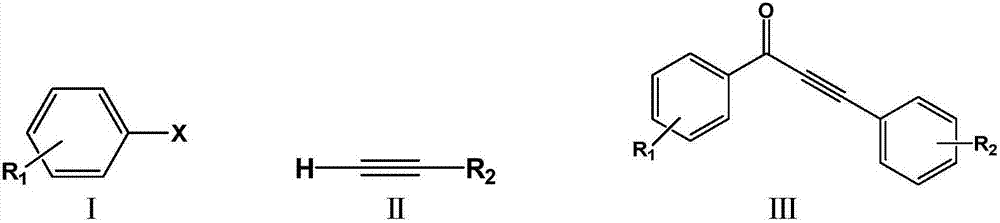
Method for preparing alpha, beta-acetylenic ketone compounds through carbon monoxide-releasing molecular carbonylation carbon-carbon bond coupling
A molecular carbon carbonylation and carbon monoxide technology, which is applied in the preparation of carbon-based compounds, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problems of harsh carbon monoxide release conditions, high requirements for reaction devices, and expensive reagents. Low requirements, good substrate applicability, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
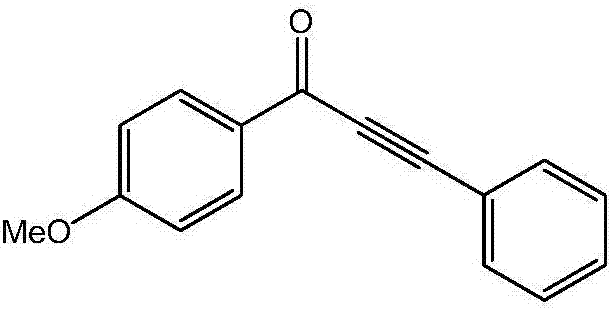
[0017] Preparation of 1-(4-methoxyphenyl)-3-phenyl-2-ethynyl-1-one of the following structural formula
[0018]
[0019] 0.1090g (0.5mmol) 4-methoxy iodobenzene, 0.0680g (0.5mmol) potassium carbonate, 0.0009g (0.005mmol) palladium chloride, 0.0058g (0.01mmol) 4,5-bisdiphenylphosphine- Add 9,9-dimethylxanthene, 3ml N,N-dimethylformamide, 55μl (0.5mmol) phenylacetylene, 67μl (0.5mmol) iron pentacarbonyl into the reaction flask, and react at 70°C for 12 hours , stop the reaction, cool down to room temperature naturally, after extraction with ethyl acetate, pickle with dilute hydrochloric acid for 3 times, then wash with water for 3 times, filter, and separate by column chromatography (the volume ratio of petroleum ether to ethyl acetate is 20:1) The mixture is the eluent) to obtain 1-(4-methoxyphenyl)-3-phenyl-2-ethynyl-1-one as a brown solid with a yield of 92%.
Embodiment 2
[0021] In Example 1, the 4-methoxy iodobenzene used was replaced with equimolar 4-methoxy bromobenzene, and the other steps were the same as in Example 1 to obtain a yellow solid 1-(4-methoxyphenyl)- 3-Phenyl-2-ethynyl-1-one in 70% yield.
Embodiment 3
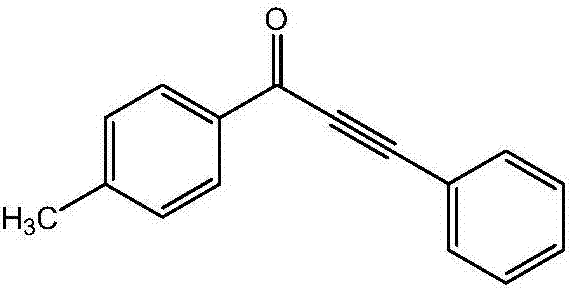
[0023] Preparation of 1-(4-methylphenyl)-3-phenyl-2-ethynyl-1-one of the following structural formula
[0024]
[0025] In Example 1, the 4-methoxy iodobenzene used was replaced with equimolar 4-methyl iodobenzene, and the other steps were the same as in Example 1 to obtain a yellow solid 1-(4-methylphenyl)-3- Phenyl-2-ethynyl-1-one in 83% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com