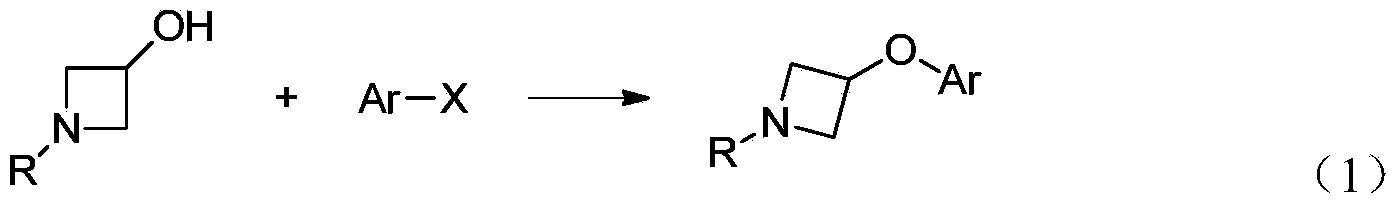
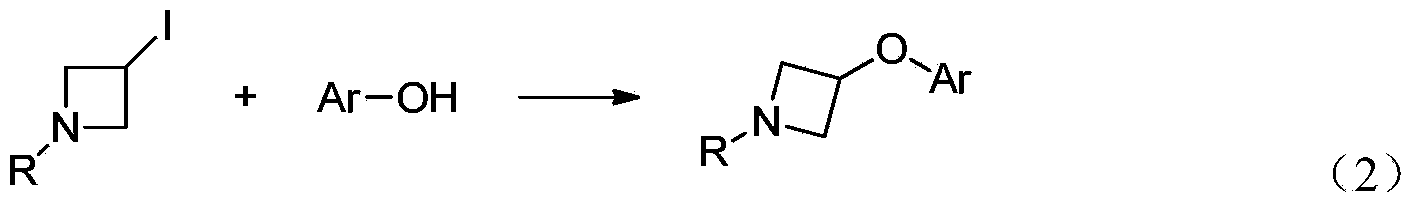
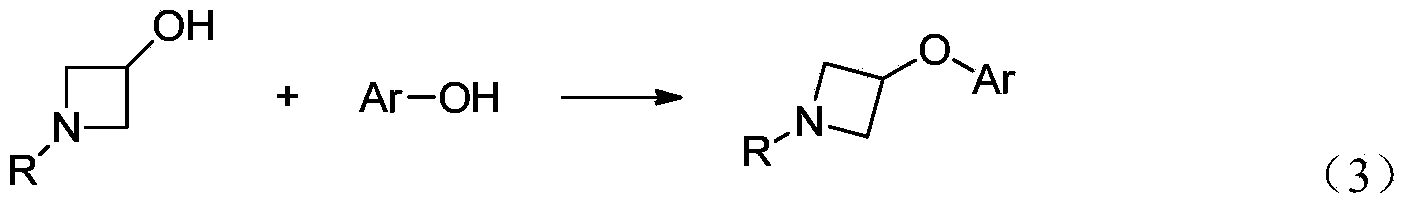
Preparation method for N-tert-butyloxycarboryl-azetidine aromatic ether/aromatic heterocyclic ether compounds
A technology of azetidine aromatic ether and tert-butoxycarbonyl, which is applied in the field of organic synthesis, can solve problems such as harsh conditions and environmental pollution, and achieve the effects of mild reaction conditions, strong reaction specificity, and good substrate applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] The preparation of N-tert-butoxycarbonyl-azetidine phenyl ether comprises the following steps:
[0034] In a dry Schlenk tube, add magneton, 1-tert-butoxycarbonyl-3-iodoazetidine (141.6 mg, 0.5 mmol), phenylboronic acid (121.9 mg, 1.0 mmol), cuprous iodide (9.5 mg, 10mol%), TMEDA (5.8mg, 10mol%), tripotassium phosphate (212.3mg, 2mmol), N,N-dimethylformamide 2mL. Under the condition of oil bath, the reaction solution was heated to 120° C., and the reaction time was 24 hours. Stop the reaction, cool to room temperature, dilute the reaction solution with ethyl acetate, wash the organic phase twice with water, once with saturated brine, and wash with MgSO 4 Dry, filter, concentrate, and evacuate. The crude product was separated by column chromatography using ethyl acetate / petroleum ether=1:100-1:80 as a developing solvent to obtain 37 mg of the target product, with a yield of 30%. The NMR characterization of the compound is as follows 1 H NMR (400MHz, CDCl ...
Embodiment 2
[0036] The preparation of N-tert-butoxycarbonyl-azetidine phenyl ether comprises the following steps:
[0037]In a dry Schlenk tube, add magneton, 1-tert-butoxycarbonyl-3-iodoazetidine (141.6 mg, 0.5 mmol), phenylboronic acid (121.9 mg, 1.0 mmol), cuprous iodide (9.5 mg, 10mol%), TMEDA (5.8mg, 10mol%), potassium carbonate (138.2mg, 2mmol), N,N-dimethylformamide 2mL. Under the condition of oil bath, the reaction solution was heated to 120° C., and the reaction time was 24 hours. Stop the reaction, cool to room temperature, dilute the reaction solution with ethyl acetate, wash the organic phase twice with water, once with saturated brine, and wash with MgSO 4 Dry, filter, concentrate, and evacuate. The crude product was separated by column chromatography using ethyl acetate / petroleum ether=1:100-1:80 as a developing solvent to obtain 22 mg of the target product with a yield of 18%.
Embodiment 3
[0039] The preparation of N-tert-butoxycarbonyl-azetidine phenyl ether comprises the following steps:
[0040] In a dry Schlenk tube, add magneton, 1-tert-butoxycarbonyl-3-iodoazetidine (141.55 mg, 0.5 mmol), phenylboronic acid (121.9 mg, 1.0 mmol), cuprous iodide (9.5 mg, 10mol%), TMEDA (5.8mg, 10mol%), sodium hydroxide (40.0mg, 2mmol), N,N-dimethylformamide 2mL. Under the condition of oil bath, the reaction solution was heated to 120° C., and the reaction time was 24 hours. Stop the reaction, cool to room temperature, dilute the reaction solution with ethyl acetate, wash the organic phase twice with water, once with saturated brine, and wash with MgSO 4 Dry, filter, concentrate, and evacuate. The crude product was separated by column chromatography using ethyl acetate / petroleum ether=1:100-1:80 as a developing solvent to obtain 21mg of the target product with a yield of 17%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com