Method for synthesizing alpha, beta-unsaturated amide compound through visible light catalysis
An amide compound, unsaturated technology, applied in the field of visible light catalytic synthesis of α, β-unsaturated amide compounds, to achieve the effect of mild reaction conditions, simple operation, and strong substrate applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
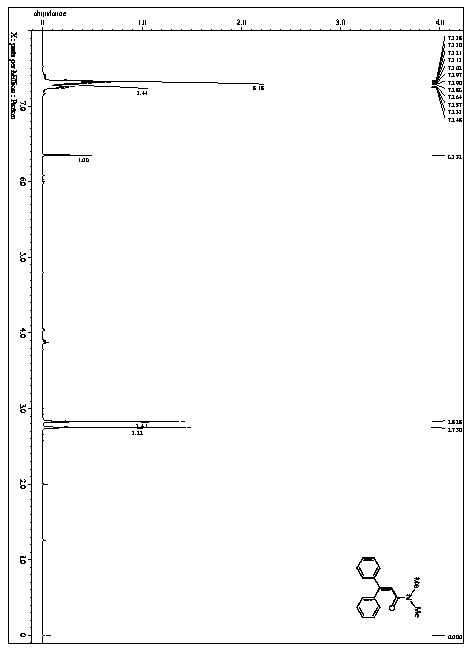
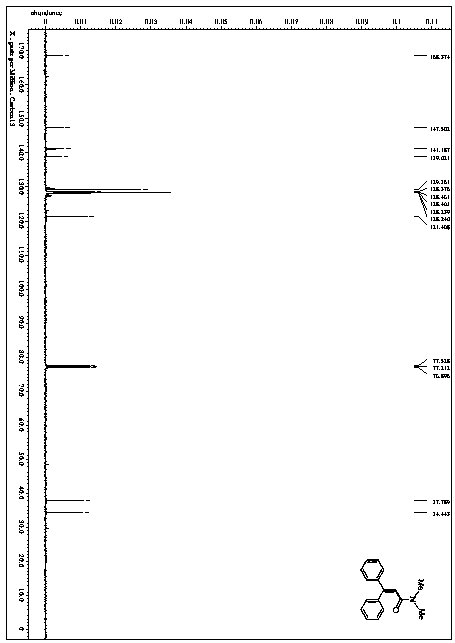
[0050] This example provides a method for the catalytic synthesis of N,N-dimethyl-3,3-diphenylacrylamide by visible light, and the synthesis route is as follows:
[0051]
[0052] The 1,2,3,4-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene is a photocatalyst, and its structural formula is:
[0053]
[0054] Wherein, Cz=carbazol-9-yl.
[0055] Described trifluoroacetic acid-(2,4,6-trimethoxyphenyl) iodine (III) benzene is an oxidizing agent, and its structural formula is:
[0056]
[0057] According to the above synthetic route, the concrete experimental steps are as follows:
[0058] 1) In a nitrogen atmosphere of 1 atmosphere, 1.80 g (10 mmol) of stilbene, 1,2,3,4-tetrakis(carbazol-9-yl)-4,6-dicyanobenzene 0.158g (0.2mmol), trifluoroacetic acid-(2,4,6-trimethoxyphenyl) iodine (III) benzene 9.68g (20mmol), N,N-dimethylformamide 200mL were sequentially added to a dry and clean in a one-mouth bottle;
[0059] 2) Under the temperature condition of 25°C, place the one-mout...
Embodiment 2
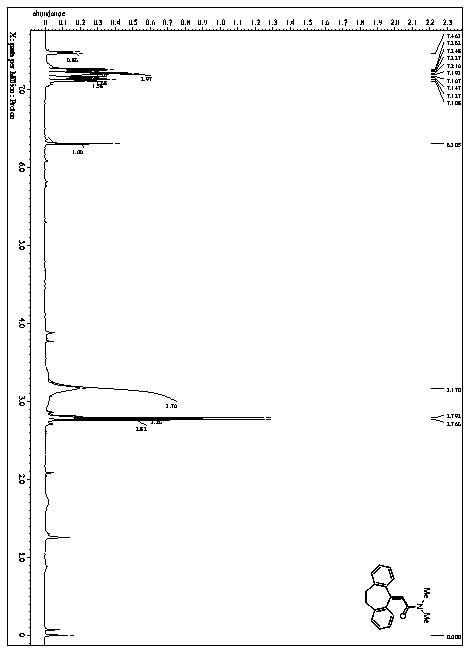
[0068] This example provides a method for the catalytic synthesis of 2-(10,11-dihydro-5H-dibenzo[a,d][7]annulene-5-methylene)-N,N-dimethylpropene The method for amide, its synthetic route is as follows:
[0069]
[0070] According to the above synthetic route, the concrete experimental steps are as follows:
[0071] 1) In a nitrogen atmosphere of 1 atmosphere, 2.06 g (10 mmol) of 5-methylene-10,11-dihydro-5H-diphenyl[a,d][7]annulene, 1,2,3 ,4-tetra(carbazol-9-yl)-4,6-dicyanobenzene 0.158g (0.2mmol), trifluoroacetic acid-(2,4,6-trimethoxyphenyl)iodo(III)benzene Add 9.68g (20mmol) and 200mL of N,N-dimethylformamide into a dry and clean single-necked bottle in turn;
[0072] 2) Under the temperature condition of 25°C, place the one-mouth bottle under a blue LED lamp with a light source power of 18W to irradiate the 5-methylene-10,11-dihydro-5H-diphenyl[ a, d][7]annulene disappears (TLC tracking), indicating that the reaction is over, and the reaction solution is obtained at...
Embodiment 3
[0081] This example provides a method for the catalytic synthesis of 3-(4-fluorophenyl)-N,N-dimethyl-3-(2-thienyl)acrylamide under visible light, and the synthesis route is as follows:
[0082]
[0083] According to the above synthetic route, the concrete experimental steps are as follows:
[0084] 1) In a nitrogen atmosphere of 1 atmosphere, 2.04g (10mmol) of 2-[1-(4-fluorophenyl)vinyl]thiophene, 1,2,3,4-tetrakis(carbazol-9-yl) -4,6-dicyanobenzene 0.158g (0.2mmol), trifluoroacetic acid-(2,4,6-trimethoxyphenyl)iodo(III)benzene 9.68g (20mmol), N,N-dimethyl Add 200mL of methyl formamide into a dry and clean single-necked bottle in turn;
[0085] 2) Under the temperature condition of 25°C, place the one-mouth bottle under a blue LED light with a light source power of 18W until the 2-[1-(4-fluorophenyl)vinyl]thiophene disappears (TLC Tracking), indicating that the reaction is over, and the reaction solution is obtained at this time, wherein the one-mouth bottle is 10-15cm awa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com