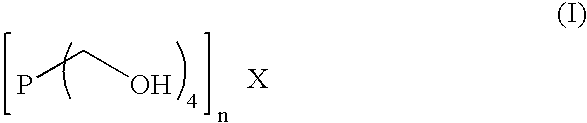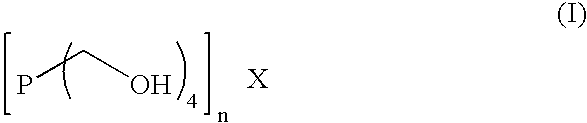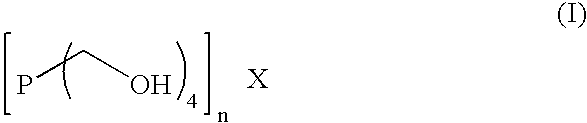Method and composition to decrease iron sulfide deposits in pipe lines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Composition Generated from Tetrakis(hydroxymethyl)phosphonium sulfate (THPS) and Ammonium Chloride
[0065] A. Tetrakis(hydroxymethyl)phosphonium sulfate (THPS) is obtained commercially as a 75-90 weight % aqueous solution with pH that varies below 4. The following procedure yielded 1000 g of a 5% aqueous composition able to complex iron sulfide. 66.6 grams of 75 weight % THPS (in water) and 0.5 grams ammonium chloride were combined, diluted with 90 grams of water, and then mixed. A sufficient amount of a 30% weight aqueous solution of sodium or potassium hydroxide was added to raise the pH to about 6.5. The total weight of the product was brought to 1000 grams by adding water, whereupon the pH was remeasured. After dilution, pH can be readjusted slightly, if necessary, to the desired value.
[0066] In this example, water can be replaced with methanol in varying amounts to produce solutions with as little as 0% water and as much as 95% water, depending upon the overall relative amounts...
example 2
Composition Generated from Tetrakis(hydroxymethyl)phosphonium sulfate (THPS) and Methylamine
[0068] Following the procedure in Example 1, a 5% by weight composition was prepared by 1) combining 6.66 g of tetrakis(hydroxymethyl)phosphonium sulfate in the form of its 75% aqueous solution by weight with enough water to make 90 mL of solution, 2) adding concentrated (12M aqueous KOH) caustic to form TRIS (95% conversion) at a pH of 7.7, 3) diluting with water to 100 mL, 4) adding 0.263 grams of methylamine, and 5) mixing. The mole ratio of TRIS to amine in this mixture is 2.6:1.
example 3
Determination of Optimum Ratio of Reactants to Iron
[0069] The following determinations demonstrate how the relative molar amounts of TRIS and an amine source affect optimum iron (II) complexation. In these determinations, iron (II) sulfate heptahydrate, a water soluble-iron (II) compound, was selected as a convenient standard iron source. TRIS was generated using the general procedures set forth in Examples 1 and 2.
[0070] The complex formed from TRIS, ammonia, and iron (II) exhibits an absorbance maximum in the vicinity of 490 nm. To find the optimum ratio of reactants, the absorbances of various compositions of the complexing components were measured. The optimal molar ratio of the components [TRIS, ammonia, iron (II)] was observed to be 20:8:5.
[0071] A similar trial using methylamine gave a complex with an absorption maximum at 473 nm, and an optimal ratio (in moles) of 26:10:5 for [TRIS, methylamine, iron (II)].
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com