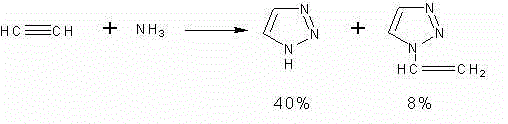
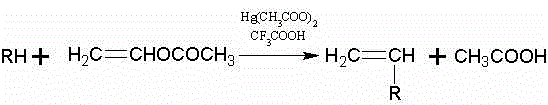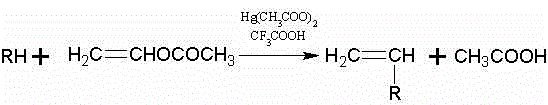Synthetic method for N-vinyl-triazole compound
A synthesis method and triazole technology are applied in the field of organic synthesis, which can solve the problems of high synthesis cost and harsh reaction conditions, and achieve the effects of good substrate applicability and easy operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1: N-vinyl 1,2,3-triazole
[0026] Add 150ml (1.62mol) of vinyl acetate, 0.2g of hydroquinone, 9.5g of mercury acetate, 9.6ml of trifluoroacetic acid, and 37.0g (0.51mol) of 1,2,3-triazole Put it into a 500ml flask with a spherical condenser, and add a stirring magnet to it, stir it strongly under normal temperature for 10-20min, then heat it, and control the reaction temperature to 60°C. The whole reaction device was ventilated with nitrogen, and the reaction was carried out under the protection of nitrogen. After reacting for 3 hours, stop heating and stirring, and let it stand for cooling. At this time, the solution was dark brown. The solution in the flask was distilled under reduced pressure, and the unreacted vinyl acetate was evaporated under reduced pressure. The remaining liquid in the flask was neutralized with saturated sodium bicarbonate solution until the pH was close to 7. The neutralized liquid was added to a separatory funnel, extracted three t...
Embodiment 2
[0029] Example 2: N-vinyl 1,2,3-triazole
[0030] Add 150ml (1.62mol) of vinyl acetate, 0.2g of hydroquinone, 9.5g of mercury acetate, 9.6ml of trifluoroacetic acid, and 37.0g (0.51mol) of 1,2,3-triazole Put it into a 500ml flask with a spherical condenser, and add a stirring magnet to it, stir it vigorously under normal temperature for 10-20min, then heat it, and control the reaction temperature to 65°C. The whole reaction device was ventilated with nitrogen, and the reaction was carried out under the protection of nitrogen. After reacting for 3 hours, stop heating and stirring, and let it stand for cooling. At this time, the solution was dark brown. The solution in the flask was distilled under reduced pressure, and the unreacted vinyl acetate was evaporated under reduced pressure. The remaining liquid in the flask was neutralized with saturated sodium bicarbonate solution until the pH was close to 7. The neutralized liquid was added to a separatory funnel, extracted three t...
Embodiment 3
[0033] Example 3: N-vinyl 1,2,3-triazole
[0034] Add 150ml (1.62mol) of vinyl acetate, 0.2g of hydroquinone, 9.5g of mercury acetate, 9.6ml of trifluoroacetic acid, and 37.0g (0.51mol) of 1,2,3-triazole Put it into a 500ml flask with a spherical condenser, and add a stirring magnet to it, stir it vigorously under normal temperature for 10-20min, then heat it, and control the reaction temperature to 70°C. The whole reaction device was ventilated with nitrogen, and the reaction was carried out under the protection of nitrogen. After reacting for 3 hours, stop heating and stirring, and let it stand for cooling. At this time, the solution was dark brown. The solution in the flask was distilled under reduced pressure, and the unreacted vinyl acetate was evaporated under reduced pressure. The remaining liquid in the flask was neutralized with saturated sodium bicarbonate solution until the pH was close to 7. The neutralized liquid was added to a separatory funnel, extracted three...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com