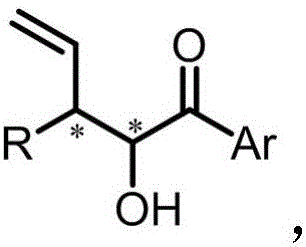
3-substituted 3-vinyl-2-hydroxy-1-aryl acetone and synthetic method thereof
A technology of aryl acetone and synthesis method, which is applied in the field of synthesis of chiral compounds, can solve the problems of poor reaction selectivity, unfavorable drug synthesis and screening, increased economic cost and time cost, etc., and achieves mild reaction conditions, shortened process, and easy synthesis low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1.8 mg of L1 ligand was added to a 5 ml reaction flask, and 0.3 ml of toluene and 12.5 μl of diethyl zinc solution (0.0125 mmol) were added thereto under nitrogen protection, and stirred at 25° C. for 30 minutes to obtain a zinc catalyst.
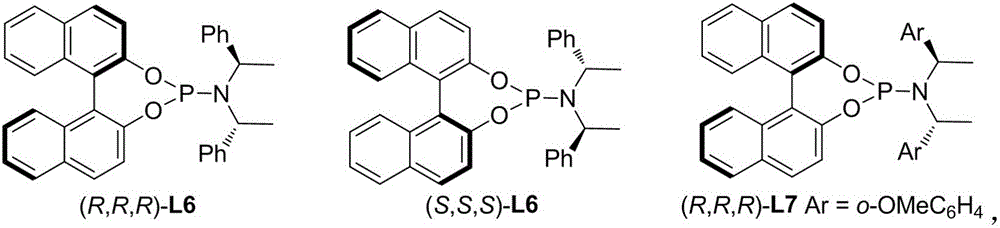
[0031] Add 3.4mg (1,5-cyclooctadiene) iridium chloride dimer and 5.4mg (R,R,R)-L6 ligand to a 10ml reaction bottle, and add 0.5ml Tetrahydrofuran and 0.5ml of n-propylamine were stirred at 50°C for 30 minutes, and the solvent was concentrated and spin-dried to obtain an iridium catalyst.
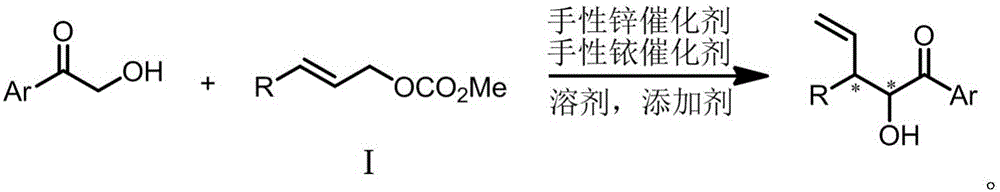
[0032] Under nitrogen protection conditions, 48.0mg cinnamyl methyl carbonate, 40.8mg 2-hydroxy-1-acetophenone, 100mg Molecular sieves, zinc catalyst, iridium catalyst and 2ml tetrahydrofuran were added into a 10ml reaction flask, and stirred at 25°C for 12 hours. The reaction solution was concentrated and spin-dried to dry the solvent, and passed through a silica gel column to obtain a purified product with a yield of 41%, a dr value of 1:1, and...
Embodiment 2
[0035]2.5 mg of L2 ligand was added to a 5 ml reaction bottle, and 0.3 ml of toluene and 12.5 μl of diethyl zinc solution (0.0125 mmol) were added thereto under nitrogen protection, and stirred at 25° C. for 30 minutes to obtain a zinc catalyst.
[0036] Add 3.4mg (1,5-cyclooctadiene) iridium chloride dimer and 5.4mg (R,R,R)-L6 ligand to a 10ml reaction bottle, and add 0.5ml Tetrahydrofuran and 0.5ml of n-propylamine were stirred at 50°C for 30 minutes, and the solvent was concentrated and spin-dried to obtain an iridium catalyst.
[0037] Under nitrogen protection conditions, 48.0mg cinnamyl methyl carbonate, 40.8mg 2-hydroxy-1-acetophenone, 100mg Molecular sieves, zinc catalyst, iridium catalyst and 2ml tetrahydrofuran were added into a 10ml reaction flask, and stirred at 25°C for 12 hours. The reaction solution was concentrated and spin-dried to dry the solvent, and passed through a silica gel column to obtain a purified product with a yield of 49%, a dr value of 1:1, and...
Embodiment 3
[0039] 3.3 mg of L3 ligand was added to a 5 ml reaction bottle, and 0.3 ml of toluene and 12.5 μl of diethyl zinc solution (0.0125 mmol) were added thereto under nitrogen protection, and stirred at 25° C. for 30 minutes to obtain a zinc catalyst.
[0040] Add 3.4mg (1,5-cyclooctadiene) iridium chloride dimer and 5.4mg (R,R,R)-L6 ligand to a 10ml reaction bottle, and add 0.5ml Tetrahydrofuran and 0.5ml of n-propylamine were stirred at 50°C for 30 minutes, and the solvent was concentrated and spin-dried to obtain an iridium catalyst.
[0041] Under nitrogen protection conditions, 48.0mg cinnamyl methyl carbonate, 40.8mg 2-hydroxy-1-acetophenone, 100mg Molecular sieves, zinc catalyst, iridium catalyst and 2ml tetrahydrofuran were added into a 10ml reaction flask, and stirred at 25°C for 12 hours. The reaction solution was concentrated and spin-dried to dry the solvent, and the purified product was obtained by passing through a silica gel column with a yield of 44%, a dr value o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com