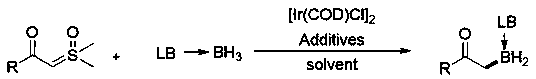Method for synthesizing alpha-borocarbonyl compound through B-H bond insertion reaction with iridium as catalyst and sulfur ylide as Carbene precursor
A carbonyl compound and insertion reaction technology, applied in the field of molecular entities - α-boronocarbonyl compounds, can solve the problems of cumbersome steps, harsh conditions, low yields, etc., and achieve broad application prospects and good substrate applicability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
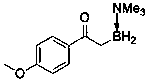
[0011] Implementation Case 1: Synthesis of Compound 1
[0012]
[0013] Add sulfur ylide (0.15 mmol), borane adduct (0.6 mmol), [Ir(COD)Cl] 2 (5 mol %) and CuF 2 (20 mol%). Then add 10ml of PhCl, stir at 60°C for 3h, and TLC detects that the reaction is complete. Then it was diluted with EA, and the solvent was removed under reduced pressure. It was separated and purified by PE / EA silica gel chromatography to obtain a white solid with a melting point of 59-61° C. and a yield of 94%.
[0014] 1 (400 MHz, Chloroform- d ) δ 8.00 (d, J = 7.2 Hz, 2H), 7.47 (t, J =7.2 Hz, 1H), 7.40 (t, J = 7.2 Hz, 2H), 2.61 (s, 9H), 2.46 (t, J = 5.2 Hz,2H); 13 C NMR (101 MHz, Chloroform- d ) δ 208.09, 138.12, 131.84, 128.79, 128.13, 52.29, 29.84; 11 B NMR (128 MHz, Chloroform- d ) δ -3.74 (t, J B-H = 101.9 Hz); HRMS (ESI) m / z Calculated value [C 11 h 18 BNNaO, M + Na] + : 214.0708; Found: 214.0710.
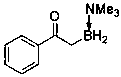
Embodiment example 2
[0015] Implementation Case 2: Synthesis of Compound 2
[0016]
[0017] Add sulfur ylide (0.15 mmol), borane adduct (0.6 mmol), [Ir(COD)Cl] 2 (5 mol %) and CuF 2 (20 mol%). Then add 10ml of PhCl, stir at 60°C for 3h, and TLC detects that the reaction is complete. Then it was diluted with EA, and the solvent was removed under reduced pressure. It was separated and purified by PE / EA silica gel chromatography to obtain a white solid with a melting point of 64-65°C and a yield of 81%.
[0018] 1 H NMR (400 MHz, Chloroform- d ) δ 7.50 (dd, J = 1.6, 0.8 Hz, 1H), 7.09(dd, J = 3.6, 0.8 Hz, 1H), 6.45 (dd, J = 3.6, 1.6 Hz, 1H), 2.61 (s, 9H), 2.30(t, J = 5.2 Hz, 2H); 13 C NMR (101 MHz, Chloroform- d ) δ 197.15, 153.66, 145.15, 116.16, 111.67, 52.34, 29.84; 11 B NMR (128 MHz, Chloroform- d ) δ -3.10 (t, J B-H = 103.0 Hz); HRMS (ESI) m / z Calculated value [C 9 h 16 BYZGR 2 , M + Na] + : 204.1172; Found: 224.1175.
Embodiment example 3
[0019] Implementation Case 3: Synthesis of Compound 3
[0020]
[0021] Add sulfur ylide (0.15 mmol), borane adduct (0.6 mmol), [Ir(COD)Cl] 2 (20 mol %) and CuF 2 (2.0 eq). Then add 10ml of PhCl, stir at 60°C for 3h, and TLC detects that the reaction is complete. Then it was diluted with EA, and the solvent was removed under reduced pressure. It was separated and purified by PE / EA silica gel chromatography to obtain a white solid with a melting point of 45-47° C. and a yield of 97%.
[0022] 1 (400 MHz, Chloroform- d ) δ 2.57 (s, 9H), 1.96 (d, J = 5.2 Hz, 2H),1.83 – 1.60 (m, 8H), 1.34 – 1.26 (m, 3H), 1.24 – 1.17 (m, 2H). 13 C NMR (101MHz, Chloroform- d ) δ 222.18, 52.33, 49.85, 29.84, 29.34, 26.24, 26.16; 11 B NMR (128 MHz, Chloroform- d ) δ -4.52 (t, J B-H = 101.3 Hz); HRMS (ESI) m / z Calculated value [C 11 h 24 BNNaO, M + Na] + :220.1849; Found: 220.1845.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com