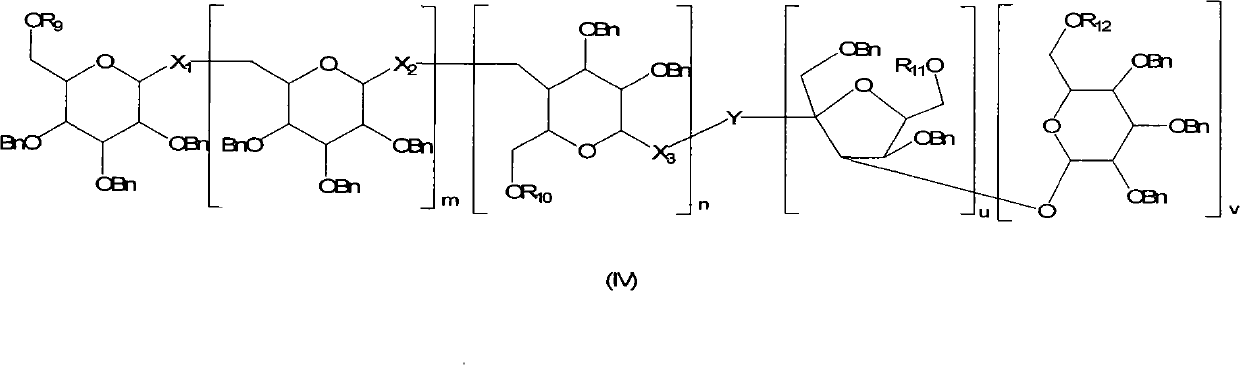
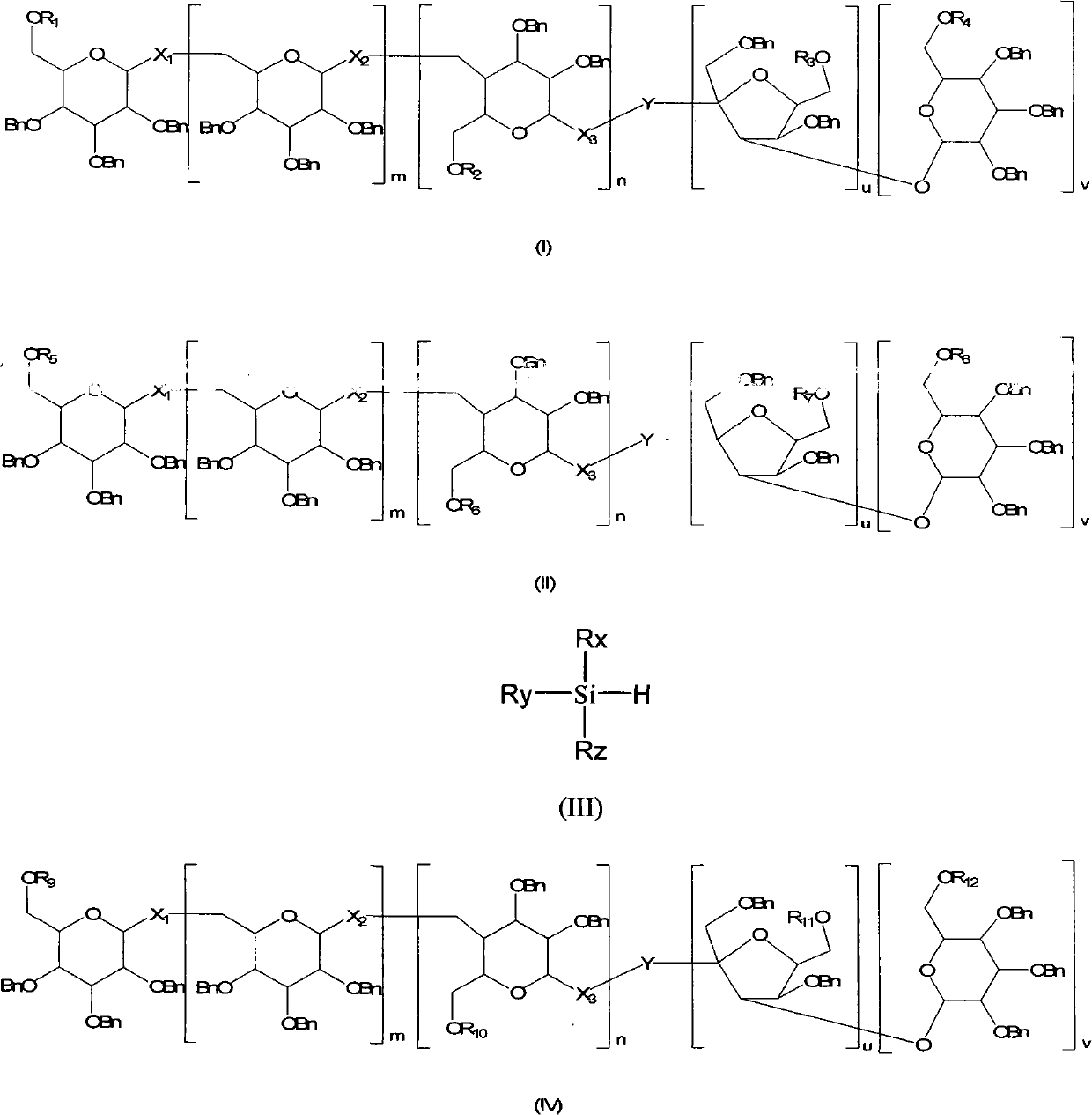
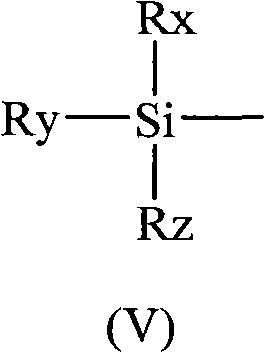Method for regioselectively removing O-benzyl protective group of sugar
A technology of benzyl and alkyl groups, applied in the field of regioselective removal of sugar O-benzyl protecting groups, can solve problems such as limiting the development and utilization of natural oligosaccharides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Preparation of methyl 2,3,4-tri-O-benzyl-triethylsilyl-α-D-mannanose (Method 1)
[0079] Under the protection of CO gas, 3.19mL (20mmol) Et 3 SiH is added to 35mg (0.1mmol) Co 2 (CO) 8 In, stirring at room temperature for 30 minutes. Then, 6 mL of anhydrous benzene was added to 554 mg (1 mmol) of methyl 2,3,4,6-tetra-O-benzyl-α-D-mannanose, and added to the above reaction solution after deoxygenation. Then it was transferred to the autoclave and reacted at 90 atm CO and 90°C for about 48 hours. After the reaction, the reaction solution was transferred to a round-bottomed flask, 0.1 mL of pyridine was added, and air was vented for about 20 minutes, and then passed through a silica gel column of about 5 cm, and then separated by a pressurized column (petroleum ether / ethyl acetate = 15: 1) Get 520 mg of colorless syrup. The yield was 90%. 1 H NMR(400MHz, C 6 D 6 )δ7.00-7.09(m, 6H), 6.77-6.90(m, 11H), 4.75(d, 1H, J=11.4Hz, -CH 2 Ph), 4.46 (d, 1H, J = 1.3 Hz, H-1), 4.42 (d, 1...
Embodiment 2
[0083] Preparation of methyl 2,3,4-tri-O-benzyl-triethylsilyl-α-D-galactopyranose
[0084] Under the protection of CO gas, 3.19mL (20mmol) Et 3 SiH is added to 35mg (0.1mmol) Co 2 (CO) 8 In, stirring at room temperature for 30 minutes. Then, 6 mL of anhydrous benzene was added to 554 mg (1 mmol) of methyl 2,3,4,6-tetra-O-benzyl-α-D-galactopyranose, and added to the above reaction solution after deoxygenation. Then it was transferred to the autoclave and reacted at 90 atm CO and 90°C for about 48 hours. After the reaction, the reaction solution was transferred to a round-bottomed flask, 0.1 mL of pyridine was added, and air was vented for about 20 minutes, and then passed through a silica gel column of about 5 cm, and then separated by a pressurized column (petroleum ether / ethyl acetate = 15: 1) 491 mg of colorless syrup is obtained. The yield was 85%. 1 H NMR(400MHz, C 6 D 6 )δ7.15-7.18(m, 2H), 7.01-7.05(m, 4H), 6.76-6.92(m, 10H), 4.88(d, 1H, J=11.3Hz, -CH 2 Ph), 4.52 (d, 1H, J...
Embodiment 3
[0086] Preparation of methyl 2,3,4-tri-O-benzyl-triethylsilyl-α-D-glucopyranose
[0087] Under the protection of CO gas, 3.19mL (20mmol) Et 3 SiH is added to 35mg (0.1mmol) Co 2 (CO) 8 In, stirring at room temperature for 30 minutes. Then, 6 mL of anhydrous benzene was added to 554 mg (1 mmol) of methyl 2,3,4,6-tetra-O-benzyl-α-D-glucopyranose, and added to the above reaction solution after deoxygenation. Then it was transferred to the autoclave and reacted at 90 atm CO and 90°C for about 48 hours. After the reaction, the reaction solution was transferred to a round-bottomed flask, 0.1 mL of pyridine was added, and air was vented for about 20 minutes, and then passed through a silica gel column of about 5 cm, and then separated by a pressurized column (petroleum ether / ethyl acetate = 15: 1) 462 mg of colorless syrup is obtained. The yield was 80%. 1 H NMR(400MHz, C 6 D 6 )δ7.05 (d, 4H, J = 7.8 Hz), 6.99 (d, 2H, J = 7.2 Hz), 6.77 690 (m, 10H), 4.76 (d, 1H, J = 11.4H, -CH 2 Ph), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com