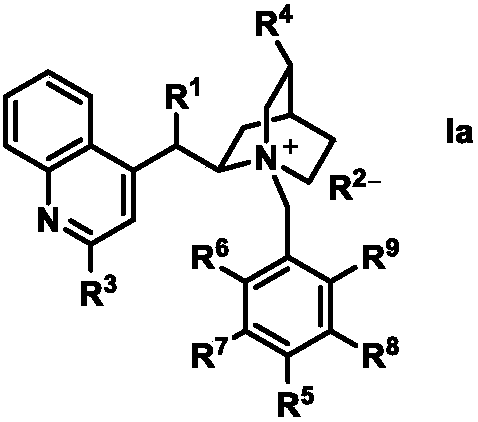
Organic catalysts having visible photocatalytic asymmetric hydroxylation photocatalysis performance, and preparation method and application thereof
An organic catalyst, a technology that catalyzes hydroxyl groups. It is applied in the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc., and can solve problems such as long reaction time and increased reaction costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
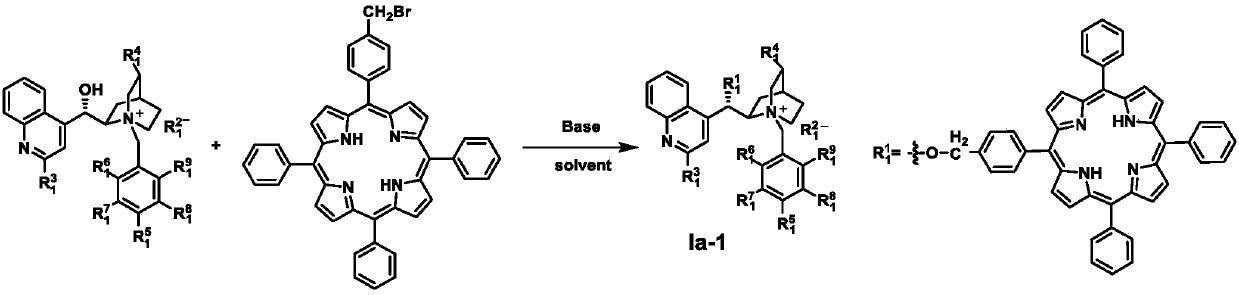
[0045] Preparation of Ia-1a
[0046]
[0047] Weigh 0.767g Cn-1 and 2.12g 5-(4-bromomethylphenyl)-10,15,20-triphenyl-21H,23H-porphyrin (TPP-1) in 50mL CH 2 Cl 2 , under the protection of nitrogen, add 1mL 50% KOH aqueous solution, stir at room temperature for 10 hours, after the reaction is completed, use 50mL water to quench the reaction, and use 3×50mLCH 2 Cl 2 Extraction, drying, spin-drying, crude product column chromatography (MeOH / EA / PE / Et 3 N=5 / 30 / 63 / 2), and 0.524 g of purple solid Ia-1a was obtained with a yield of 38%. 1 H NMR (400MHz, DMSO-d6) δ8.84(s, 4H), 8.72(d, J=4.7Hz, 2H), 8.62(s, 2H), 8.58–8.46(m, 4H), 8.29(m, 3H), 8.21(m, 6H), 8.04(d, J=7.7Hz, 2H), 8.01–7.94(m, 4H), 7.87(m, 9H), 7.74–7.67(m, 1H), 7.59(t ,J=7.4Hz,1H),7.49(t,J=7.7Hz,1H),6.65(s,1H),6.19(d,J=8.5Hz,1H),5.33(d,J=14.3Hz,2H ),5.26–5.17(m,2H),5.09(d,J=11.9Hz,1H),4.67(d,J=12.1Hz,1H),4.37(s,1H),4.02(d,J=15.0Hz ,1H),3.77–3.70(m,1H),3.38(s,2H),2.79–2.65(m,2H),1.81(s,1H),1.46–1.35(m,1H),-2.94(s, ...
Embodiment 2
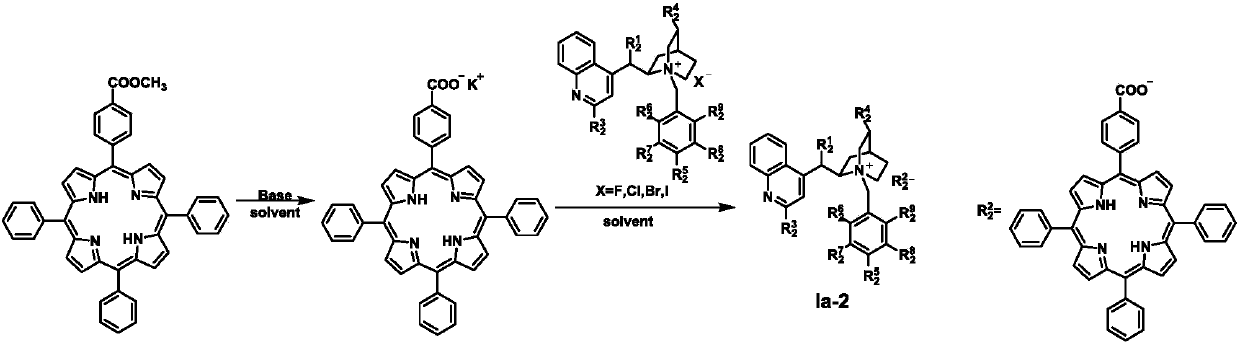
[0049] Preparation of Ia-2a
[0050]
[0051] Weigh 0.71g of 5-(4-methoxycarbonylphenyl)-10,15,20-triphenyl-21H,23H-porphyrin (TPP-2) and dissolve it in 80mLTHF, add 20mL2MKOH, heat to reflux overnight, cool to room temperature, add 100mL water-extracted aqueous solution containing 5-(4-formylphenyl)-10,15,20-triphenyl-21H,23H-porphyrin potassium salt (TPP-3), the water phase is not Post-processing proceeds directly to the next step.
[0052] Add 0.77g Cn-1 and 200mL CHCl to the aqueous solution containing TPP-3 3 , stirred at room temperature for 1 h, separated and collected the organic phase, washed the organic phase with 3×50 mL of water, dried, and rotary evaporated to obtain 1.31 g of purple solid Ia-2a with a yield of 97%. 1 H NMR (400MHz, DMSO-d6) δ8.82 (s, 8H), 8.54 (d, J = 8.6Hz, 3H), 8.39 (s, 1H), 8.19 (d, J = 15.8Hz, 12H), 7.94 (d,J=18.6Hz,5H),7.82(s,10H),7.06(s,1H),6.91(s,2H),6.61(s,1H),5.17(d,J=12.1Hz,1H) ,5.04(d,J=12.6Hz,1H),4.44(s,1H),3.98(s,2H),3.73(m,1H...
Embodiment 3
[0054] Preparation of Ia-3a
[0055]
[0056] Weigh 0.75gCn-3, 1.78gTPP-4 and 0.55gK 2 CO 3 and 0.12gPd(PPh 3 ) 4 in 50mLMeOH and 75mLPhCH 3 , under the protection of nitrogen, heated to 80 ° C, the reaction 12h. Cool to room temperature, add 200mL CH 2 Cl 2 , using 200mL10%Na 2 CO 3 Solution washing and 3×50mL water washing, organic phase using anhydrous Na 2 SO 4 Drying, rotary evaporation, the crude product was separated by column chromatography (MeOH / CH 2 Cl 2 =1 / 20), to obtain 1.27g purple solid Cn-4, yield 70%. 1 H NMR (400MHz, DMSO-d6) δ8.95(d, J=4.8Hz, 2H), 8.83(d, J=9.7Hz, 6H), 8.70(d, J=7.9Hz, 2H), 8.53(d ,J=9.3Hz,2H),8.41(d,J=7.8Hz,2H),8.28–8.15(m,7H),7.92–7.70(m,11H),6.55(m,3H),3.69(s, 1H), 3.13(s, 1H), 1.87(s, 1H), 1.75(s, 2H), 1.58(m, 2H), 1.37–1.27(m, 2H), 1.14(t, J=7.5Hz, 5H ),0.88(t,J=7.3Hz,3H),0.79(t,J=7.2Hz,2H),-2.91(s,2H).
[0057] Weigh 0.636g Cn-4 and 0.276g 3,5-dibromobenzyl bromide in 10mLTHF, heat to reflux, react overnight, cool to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com