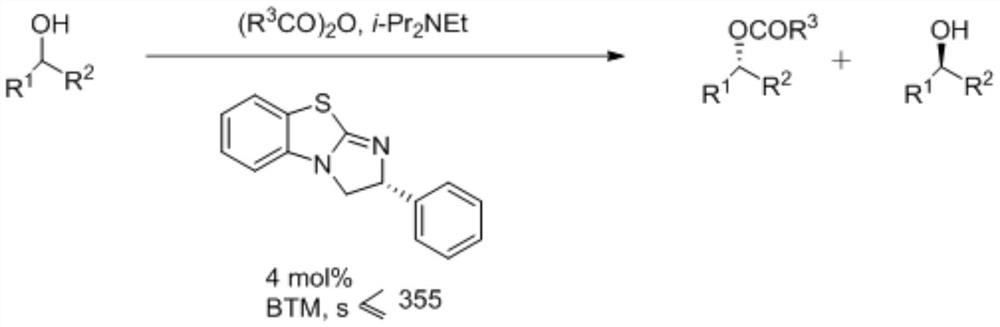
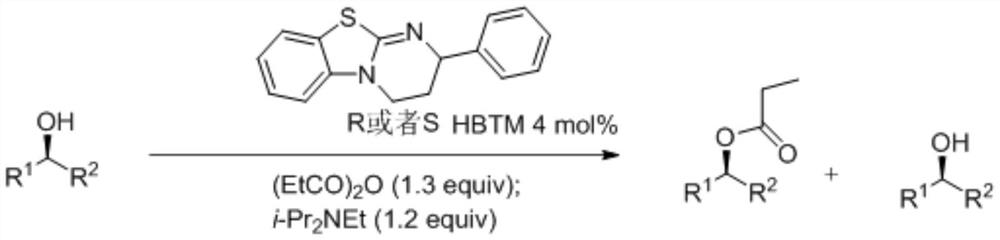
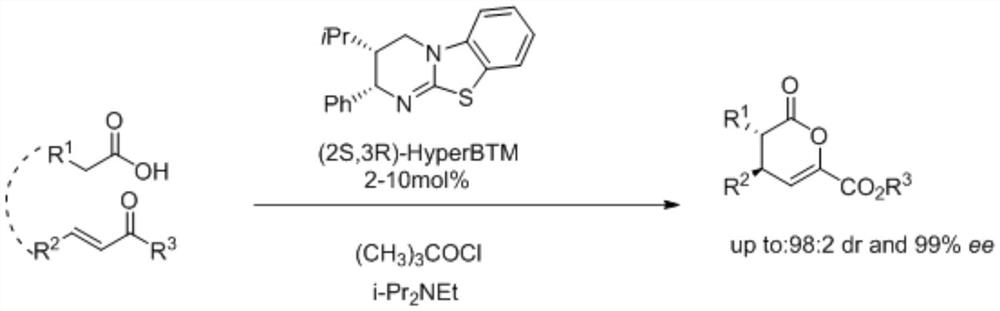
A kind of synthetic method of isothiourea catalyst
A synthetic method and technology of isothiourea, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve the problems of toxicity and low yield of multi-step synthesis , to achieve the effects of wide application prospects, high yield, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The synthesis of embodiment 1 compound 2 (o-nitrophenyl tert-butyl sulfide)
[0062] 2.8g (0.02mol) of o-fluoronitrobenzene (compound 1) was dissolved in 40ml of DMF solvent, 4.3g (0.03mol) of potassium carbonate solid was added, the temperature was raised to 80°C, and 2.0g (0.022mol) of tert-butyl Thiol, continue to react for 2h. After the reaction was completed, 100 ml of water and 100 ml of dichloromethane were added for extraction, and the organic phase was washed twice with 100 ml of water. The organic phase was concentrated to obtain 4.2 g (0.0199 mol) of compound 2 (o-nitrophenyl tert-butyl sulfide), with a yield of 99%. ( 1 H NMR (400MHz, CDCl 3 )δ7.71(dd, J=7.6,1.5Hz,1H),7.66(dd,J=7.7,1.6Hz,1H),7.49(dqd,J=15.0,7.5,1.6Hz,2H),1.31(s ,9H)).
Embodiment 2
[0063] The synthesis of embodiment 2 compound 3 (o-aminophenyl tert-butyl sulfide)
[0064] 4.2 g (0.0199 mol) of compound 2 (o-nitrophenyl tert-butyl sulfide) was dissolved in methanol, under nitrogen protection, 400 mg of Raney nickel was added, under hydrogen, reacted at normal temperature and pressure for 4 hours, suction filtered, and the filtrate was concentrated to obtain 3.4 g (0.0188mol) compound 3 (o-aminophenyl tert-butyl sulfide), yield 94%.
Embodiment 3
[0065] The synthesis of embodiment 3 compound 4 (o-aminophenyl tert-butyl sulfoxide)
[0066] 1.8g (0.01mol) of compound 3 (o-aminophenyl tert-butyl sulfide) was dissolved in 20ml of dichloromethane solution, cooled to -10°C, m-CPBA solution (2.1g (0.012mol) dissolved in 20ml of dichloromethane Chloromethane), after the dropwise addition, reacted for half an hour, suction filtered, the filtrate was washed twice with 40ml saturated sodium sulfite, and washed once with 40ml saturated sodium carbonate. The organic phase was dried and concentrated over sodium sulfate, and separated on a silica gel column (petroleum ether: ethyl acetate = 4:1) to obtain 1.8 g (0.009 mol) of compound 4 (o-aminophenyl tert-butyl sulfoxide), with a yield of 90%. ( 1 H NMR (400MHz, CDCl 3 )δ7.24–7.17(m,1H),7.10(d,J=7.0Hz,1H),6.71(t,J=7.5Hz,1H),6.63(d,J=8.2Hz,1H),5.15( s,2H),1.31(s,9H). 13 C NMR (100MHz, CDCl 3 )δ149.4, 131.8, 128.8, 117.9, 117.5, 116.4, 58.8, 23.5. HRMS (ESI) m / z calcd for C 10 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com